Abstract
Background
Excessive autophagy and apoptosis of the interstitial cells of Cajal (ICC) have been identified in gastrointestinal (GI) motility disorders including slow transit constipation (STC). MicroRNA 222 (miR-222) has been shown to affect GI motility. This study aimed to explore whether miR-222 influences apoptosis and excessive autophagy of isolated ICC.
Methods
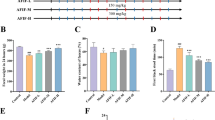
miR-222, c-kit, and stem cell factor (SCF) were evaluated in colon tissues in STC rats compared with normal control by qRT-PCR and western blot analysis. The condition of autophagy of colon tissue was observed by transmission electron microscope. ICC were isolated from the colon of STC rats. Cell Counting Kit-8 (CCK-8) assay and wound healing assay were carried out to examine the cell viability and migration rate. Cell apoptosis was detected by terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) and Annexin V-Flourescein Isothiocyanate/Propidine Iodide (FITC/PI) apoptosis detection kit. Western blot analysis was performed to detect the c-kit and SCF expression; apoptosis-related proteins Bcl-2, Bax, caspase-3, and pro-caspase-3; and autophagy-related proteins LC3B and Beclin-1. The connection between miR-222 and c-kit was detected by bioinformatics and luciferase activity analysis.
Results
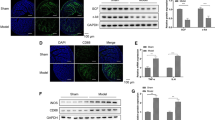
miR-222 expression was significantly higher, whereas c-kit and SCF expressions were markedly lower in STC rats’ colon tissue compared with normal control. Meanwhile, STC rats exhibited excessive autophagy in colon tissue than normal control. Inhibition of miR-222 expression promoted cell proliferation as well as migration and inhibited autophagy, whereas upregulation of miR-222 had the opposite effect. In addition, miR-222 upregulation induced apoptosis and excessive autophagy compared with normal controls (NC). Western blot analysis showed that miR-222 overexpression caused decreased c-kit and SCF protein levels compared with NC. Bioinformatics and luciferase activity analysis revealed that miR-222 could be a predictive regulator of c-kit.
Conclusion
miR-222 induces apoptosis and excessive autophagy of ICC and may serve as potential biomarker for ICC loss in STC.








Similar content being viewed by others
References
Walia R, Mahajan L, Steffen R. Recent advances in chronic constipation. Curr Opin Pediatr. 2009;21:661–6.
Bharucha AE, Pemberton JH, Locke GR 3rd. American Gastroenterological Association technical review on constipation. Gastroenterology. 2013;144:218–38.
Wong SW, Lubowski DZ. Slow-transit constipation: evaluation and treatment. ANZ J Surg. 2007;77:320–8.
Sailer M. Slow transit constipation. Zentralbl Chir. 2019;144:179–89.
Ward SM, Sanders KM. Physiology and pathophysiology of the interstitial cell of Cajal: from bench to bedside. I. Functional development and plasticity of interstitial cells of Cajal networks. Am J Physiol Gastrointest Liver Physiol. 2001;281:G602–11.
Huizinga JD, Thuneberg L, Klüppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–9.
Yu CS, Kim HC, Hong HK, et al. Evaluation of myenteric ganglion cells and interstitial cells of Cajal in patients with chronic idiopathic constipation. Int J Color Dis. 2002;17:253–8.
Wedel T, Spiegler J, Soellner S, et al. Enteric nerves and interstitial cells of Cajal are altered in patients with slow-transit constipation and megacolon. Gastroenterology. 2002;123:1459–67.
Negreanu LM, Assor P, Mateescu B, Cirstoiu C. Interstitial cells of Cajal in the gut--a gastroenterologist's point of view. World J Gastroenterol. 2008;14:6285–8.
Brennecke J, Hipfner DR, Stark A, Russell RB, Cohen SM. Bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell. 2003;113:25–36.
Poy MN, Eliasson L, Krutzfeldt J, et al. A pancreatic islet-specific microRNA regulates insulin secretion. Nature. 2004;432:226–30.
Deng Y, Zhou X, Xiang X, Ou Y, He J. Effect of miRNA-19a on gastrointestinal motility in rats with functional dyspepsia. Exp Ther Med. 2018;15:4875–9.
Zhao S, Chen Q, Kang X, Kong B, Wang Z. Aberrantly expressed genes and miRNAs in slow transit constipation based on RNA-Seq analysis. Biomed Res Int. 2018;2018:2617432.
Mazzone A, Strege PR, Gibbons SJ, et al. microRNA overexpression in slow transit constipation leads to reduced Na V 1.5 current and altered smooth muscle contractility. Gut. 2020;69:868–76.
Weicheng L, Qiulei Z, Shu L, et al. The relationship between colonic macrophages and microRNA-128 in the pathogenesis of slow transit constipation. Dig Dis Sci. 2015;60:2304–15.
Yu C, Zang L, Feng B, et al. Co-expression network analysis identified specific miRNAs and genes in association with slow-transit constipation. Mol Med Rep. 2020;22:4696–706.
Matsuzaki J, Suzuki H. Role of microRNAs-221/222 in digestive systems. J Clin Med. 2015;4:1566–77.
Luo F, Zhou J, Wang S, Sun Z, Han Q, Bai C. microRNA-222 promotes colorectal cancer cell migration and invasion by targeting MST3. FEBS Open Bio. 2019;9:901–13.
Evangelista AM, Deschamps AM, Liu D, Raghavachari N, Murphy E. miR-222 contributes to sex-dimorphic cardiac eNOS expression via ets-1. Physiol Genomics. 2013;45:493–8.
Vannucchi MG, Corsani L, Bani D, Faussone-Pellegrini MS. Myenteric neurons and interstitial cells of Cajal of mouse colon express several nitric oxide synthase isoforms. Neurosci Lett. 2002;326:191–5.
Shan JJ, Zhang Y, Diao YL, Qu WS, Zhao XN. Effect of an antidiabetic polysaccharide from Inula japonica on constipation in normal and two models of experimental constipated mice. Phytother Res. 2010;24:1734–8.
Ren K, Yong C, Yuan H, Cao B, Zhao K, Wang J. TNF-α inhibits SCF, ghrelin, and substance P expressions through the NF-κB pathway activation in interstitial cells of Cajal. Braz J Med Biol Res. 2018;51:e7065.
Bai X, Geng J, Li X, Yang F, Tian J. VEGF-A inhibition ameliorates podocyte apoptosis via repression of activating protein 1 in diabetes. Am J Nephrol. 2014;40:523–34.
Kitamura Y, Hirota S, Nishida T. A loss-of-function mutation of c-kit results in depletion of mast cells and interstitial cells of Cajal, while its gain of function mutation results in their oncogenesis. Mutat Res. 2001;477:165–71.
Lecoin L, Gabella G, Le Douarin N. Origin of the c-kit-positive interstitial cells in the avian bowel. Development. 1996;122:725–33.
Rich A, Miller SM, Gibbons SJ, Malysz J, Szurszewski JH, Farrugia G. Local presentation of Steel factor increases expression of c-kit immunoreactive interstitial cells of Cajal in culture. Am J Physiol Gastrointest Liver Physiol. 2003;284:G313–20.
Tong WD, Liu BH, Zhang LY, Xiong RP, Liu P, Zhang SB. Expression of c-kit messenger ribonucleic acid and c-kit protein in sigmoid colon of patients with slow transit constipation. Int J Colorectal Dis. 2005;20:363–7.
Lee JI, Park H, Kamm MA, Talbot IC. Decreased density of interstitial cells of Cajal and neuronal cells in patients with slow-transit constipation and acquired megacolon. J Gastroenterol Hepatol. 2005;20:1292–8.
Su Z, Yang Z, Xu Y, Chen Y, Yu Q. MicroRNAs in apoptosis, autophagy and necroptosis. Oncotarget. 2015;6:8474–90.
Faussone-Pellegrini MS. Interstitial cells of Cajal: once negligible players, now blazing protagonists. Ital J Anat Embryol. 2005;110:11–31.
Zhang LM, Zeng LJ, Deng J, et al. Investigation of autophagy and differentiation of myenteric interstitial cells of Cajal in the pathogenesis of gastric motility disorders in rats with functional dyspepsia. Biotechnol Appl Biochem. 2018;65:533–9.
Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42.
Kourtis N, Tavernarakis N. Autophagy and cell death in model organisms. Cell Death Differ. 2009;16:21–30.
Li L, Tan J, Miao Y, Lei P, Zhang Q. ROS and autophagy: Interactions and molecular regulatory mechanisms. Cell Mol Neurobiol. 2015;35:615–21.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
HZ, YJL, ZCC, and GQF declare that they have no competing interests.
Ethics statement
The study was performed conforming to the Helsinki declaration of 1975, as revised in 2000 and 2008 concerning human and animal rights, and the authors followed the policy concerning informed consent as shown on Springer.com.
Disclaimer
The authors are solely responsible for the data and the contents of the paper. In no way, the Honorary Editor-in-Chief, Editorial Board Members, the Indian Society of Gastroenterology or the printer/publishers are responsible for the results/findings and content of this article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zheng, H., Liu, YJ., Chen, ZC. et al. miR-222 regulates cell growth, apoptosis, and autophagy of interstitial cells of Cajal isolated from slow transit constipation rats by targeting c-kit. Indian J Gastroenterol 40, 198–208 (2021). https://doi.org/10.1007/s12664-020-01143-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12664-020-01143-7