Abstract
Introduction
Sofosbuvir (SOF) and daclatasvir (DCV) regimens are recommended for all genotypes of hepatitis C virus (HCV) infection. However, DCV accessibility is still low in several low– and middle-income countries. Ribavirin (RBV) is more affordable and has been known for chronic HCV treatment along with SOF or interferon. The aim of this study was to assess the efficacy of SOF + RBV and SOF + DCV regimens for treatment of chronic HCV in Indonesia.
Methods
We conducted a retrospective study among patients with chronic HCV who were treated with SOF. Data on SOV + RBV were collected from 2015 to 2016, while those on SOF + DCV were collected from 2016 to 2017. The baseline characteristics were recorded from the medical record unit in Cipto Mangunkusumo General Hospital, Jakarta, Indonesia. The primary outcome was the achievement of sustained virological response at 12 weeks (SVR12).
Results
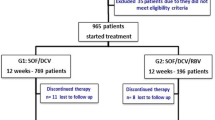
Of 309 patients, 64.4% (199/309) had genotype 1 infections, 29.8% (92/309) had cirrhosis, and 4.9% (15/309) had co-infection with human immunodeficiency virus (HIV). At the end of treatment (EOT), 99.3% (136/137) patients in the SOF + RBV group and 99.4% (164/165) in SOF + DCV group had no detectable viral load. The criterion for SVR12 was met in 90.8% (109/120) patients in SOF + RBV regimen and 98.2% (108/110) in SOF + DCV regimen. Among patients with cirrhosis, 84.4% (38/45) patients and 100% (27/27) achieved SVR12 in the SOF + RBV and SOF + DCV groups, respectively.
Conclusion
SOF + DCV regimen had higher SVR rates compared to SOF + RBV regimen (p = 0.034). However, both the regimens showed an impressive outcome, with overall SVR12 rates above 90%, irrespective of presence of cirrhosis and HCV genotype. In non-structural protein 5A inhibitor limited setting, SOF + RBV regimen still can be used as treatment for HCV infection, particularly in non-cirrhotic patients.


Similar content being viewed by others
References
World Health Organization. Global hepatitis report 2017. Geneva: World Health Organization; 2017.
Muljono DH. Epidemiology of hepatitis B and C in Republic of Indonesia. Euroasian J Hepatogastroenterol. 2017;7:55–9.
Utama A, Tania NP, Dhenni R, et al. Genotype diversity of hepatitis C virus (HCV) in HCV-associated liver disease patients in Indonesia. Liver Int. 2010;30:1152–60.
Akbar N, Sulaiman A, Gani RA, et al. Efficacy and safety of in-Asia-manufactured interferon alpha-2b in combination with ribavirin for therapy of naïve chronic hepatitis C patients: a multicenter, prospective, open-label trial. Indones J Gastroenterol Hepatol Dig Endosc. 2009;10:7–13.
European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2016. J Hepatol. 2017;66:153–94.
Bhatia HK, Singh H, Grewal N, Natt NK. Sofosbuvir: a novel treatment option for chronic hepatitis C infection. J Pharmacol Pharmacother. 2014;5:278–84.
Te HS, Randall G, Jensen DM. Mechanism of action of ribavirin in the treatment of chronic hepatitis C. Gastroenterol Hepatol (NY). 2007;3:218–25.
Lee C. Daclatasvir: potential role in hepatitis C. Drug Des Devel Ther. 2013;7:1223–33.
World Health Organization. Progress report on access to hepatitis C treatment: focus on overcoming barriers in low- and middle-income countries. Geneva: World Health Organization; 2018.
Omata M, Kanda T, Wei L, et al. APASL consensus statements and recommendation on treatment of hepatitis C. Hepatol Int. 2016;10:702–26.
Berden FA, Aaldering BR, Groenewoud H, IntHout J, Kievit W, Drenth JP. Identification of the best direct-acting antiviral regimen for patients with hepatitis C virus genotype 3 infection: A systematic review and network meta-analysis. Clin Gastroenterol Hepatol. 2017;15:349–59.
Osinusi A, Meissner EG, Lee YJ, et al. Efficacy of sofosbuvir and ribavirin for treatment of hepatitis C genotype-1 in an inner city population: virus and host factors that predict relapse: a randomized controlled trial. JAMA. 2013;310:804–11.
Omata M, Nishiguchi S, Ueno Y, et al. Sofosbuvir plus ribavirin in Japanese patients with chronic genotype 2 HCV infection: an open-label, phase 3 trial. J Viral Hepat. 2014;21:762–8.
Zeuzem S, Dusheiko GM, Salupere R, et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med. 2014;370:1993–2001.
Ruane PJ, Ain D, Stryker R, et al. Sofosbuvir plus ribavirin for the treatment of chronic genotype 4 hepatitis C virus infection in patients of Egyptian ancestry. J Hepatol. 2015;62:1040–6.
Sulkowski MS, Naggie S, Lalezari J, Fessel WJ, Mounzer K, Shuhart M, et al. Sofosbuvir and ribavirin for hepatitis C in patients with HIV coinfection. JAMA. 2014;312:353–61.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
JK, RAG, IH, ASS, CRAL, COMJ, KFK, SHHN, and SZ declare that they have no conflict of interest.
Ethics statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Disclaimer
The authors are solely responsible for the data and the content of the paper. In no way the Honorary Editor-in-Chief, Editorial Board Members, or the printer/publishers are responsible for the results/findings and content of this article.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kurniawan, J., Gani, R.A., Hasan, I. et al. Comparative efficacy of sofosbuvir-ribavirin versus sofosbuvir-daclatasvir for treatment of chronic hepatitis C in an area with limited NS5A inhibitor availability. Indian J Gastroenterol 37, 520–525 (2018). https://doi.org/10.1007/s12664-018-0921-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12664-018-0921-2