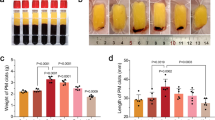
Abstract
Background/Purpose
The present study aimed to investigate plastic tubes without additives as alternatives to glass and silica-coated plastic tubes, in the production of PRF membranes.
Materials and Methods
Nine blood samples were collected from eight volunteers (n = 8) separated into three groups, according to tube material: glass, silica-coated plastic, and plastic without additives. In each group, the samples were centrifuged using different relative centrifugation forces: L-PRF (700 g/12 min), A-PRF (200 g/14 min), and A-PRF + (200 g/8 min). The generated membranes were evaluated by histomorphometry, considering the fibrin network, platelet aggregates, and cellular morphology, by light microscopy. The ultrastructural cellular morphology integrity was evaluated by transmission electron microscopy.
Results
The L-PRF (p < 0.019) and A-PRF (p < 0.001) membranes showed a significantly lower fibrin network density in plastic tubes without additives compared to glass and silica-coated plastic tubes. Plastic tubes without additives revealed a significantly higher platelet percentage, regardless of the protocol (p < 0.005). In all groups, TEM analysis showed preserved normal morphological ultrastructure, maintaining the integrity of cellular components.
Conclusion
Plastic tubes without additives offer a viable alternative for producing PRF membranes. They exhibited a higher platelet density and demonstrated fibrin network and cellular morphology similar to those of glass and silica-coated plastic tubes, irrespective of the centrifugation protocol.


Similar content being viewed by others
References
Choukroun J, Diss A, Simonpieri A et al (2006) Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 101(3):e56–e60. https://doi.org/10.1016/j.tripleo.2005.07.011
Dohan DM, Choukroun J, Diss A et al (2006) Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 101(3):e45–e50. https://doi.org/10.1016/j.tripleo.2005.07.009
Dohan DM, Choukroun J, Diss A et al (2006) Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part III: leucocyte activation: a new feature for platelet concentrates? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 101(3):e51–e55. https://doi.org/10.1016/j.tripleo.2005.07.010
Choukroun J, Adda F, Schoeffler C, Vervelle A (2000) Une opportunité en paro-implantologie: le PRF. Implantodontie 42:55–62
Dohan DM, Choukroun J, Diss A et al (2006) Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 101(3):e37–e44. https://doi.org/10.1016/j.tripleo.2005.07.008
Choukroun J, Ghanaati S (2018) Reduction of relative centrifugation force within injectable platelet-rich-fibrin (PRF) concentrates advances patients’ own inflammatory cells, platelets and growth factors: the first introduction to the low speed centrifugation concept. Eur J Trauma Emerg Surg 44(1):87–95. https://doi.org/10.1007/s00068-017-0767-9
Fujioka-Kobayashi M, Miron RJ, Hernandez M, Kandalam U, Zhang Y, Choukroun J (2017) Optimized platelet-rich fibrin with the low-speed concept: growth factor release, biocompatibility, and cellular response. J Periodontol 88(1):112–121. https://doi.org/10.1902/jop.2016.160443
Miron RJ, Fujioka-Kobayashi M, Hernandez M et al (2017) Injectable platelet rich fibrin (i-PRF): opportunities in regenerative dentistry? Clin Oral Investig 21(8):2619–2627. https://doi.org/10.1007/s00784-017-2063-9
Miron RJ, Xu H, Chai J et al (2020) Comparison of platelet-rich fibrin (PRF) produced using 3 commercially available centrifuges at both high (~ 700 g) and low (~ 200 g) relative centrifugation forces. Clin Oral Investig 24(3):1171–1182. https://doi.org/10.1007/s00784-019-02981-2
Kratz A, Stanganelli N, Van Cott EM (2006) A comparison of glass and plastic blood collection tubes for routine and specialized coagulation assays: a comprehensive study. Arch Pathol Lab Med 130(1):39–44. https://doi.org/10.5858/2006-130-39-ACOGAP
Tsujino T, Masuki H, Nakamura M et al (2019) Striking differences in platelet distribution between advanced-platelet-rich fibrin and concentrated growth factors: effects of silica-containing plastic tubes. J Funct Biomater 10(3):43. https://doi.org/10.3390/jfb10030043
Tsujino T, Takahashi A, Yamaguchi S et al (2019) Evidence for contamination of silica microparticles in advanced platelet-rich fibrin matrices prepared using silica-coated plastic tubes. Biomedicines 7(2):45. https://doi.org/10.3390/biomedicines7020045
Yamaguchi S, Aizawa H, Sato A et al (2020) Concentrated growth factor matrices prepared using silica-coated plastic tubes are distinguishable from those prepared using glass tubes in platelet distribution: application of a novel near-infrared imaging-based quantitative technique. Front Bioeng Biotechnol 8:600. https://doi.org/10.3389/fbioe.2020.00600
Miron RJ, Kawase T, Dham A, Zhang Y, Fujioka-Kobayashi M, Sculean A (2021) A technical note on contamination from PRF tubes containing silica and silicone. BMC Oral Health 21(1):135. https://doi.org/10.1186/s12903-021-01497-0
Chen L, Liu J, Zhang Y et al (2018) The toxicity of silica nanoparticles to the immune system. Nanomedicine (Lond) 13(15):1939–1962. https://doi.org/10.2217/nnm-2018-0076
Sharma N, Jha S (2020) Amorphous nanosilica induced toxicity, inflammation and innate immune responses: a critical review. Toxicology 441:152519. https://doi.org/10.1016/j.tox.2020.152519
Murugadoss S, Lison D, Godderis L et al (2017) Toxicology of silica nanoparticles: an update. Arch Toxicol 91(9):2967–3010. https://doi.org/10.1007/s00204-017-1993-y
Yazdimamaghani M, Moos PJ, Dobrovolskaia MA, Ghandehari H (2019) Genotoxicity of amorphous silica nanoparticles: status and prospects. Nanomedicine 16:106–125. https://doi.org/10.1016/j.nano.2018.11.013
Masuki H, Isobe K, Kawabata H et al (2020) Acute cytotoxic effects of silica microparticles used for coating of plastic blood-collection tubes on human periosteal cells. Odontology 108(4):545–552. https://doi.org/10.1007/s10266-020-00486-z
Margolis J (1957) Initiation of blood coagulation by glass and related surfaces. J Physiol 137(1):95–109. https://doi.org/10.1113/jphysiol.1957.sp005799
Golas A, Parhi P, Dimachkie ZO, Siedlecki CA, Vogler EA (2010) Surface-energy dependent contact activation of blood factor XII. Biomaterials 31(6):1068–1079. https://doi.org/10.1016/j.biomaterials.2009.10.039
Saboia-Dantas CJ, Limirio PHJO, Costa MDMA, Linhares CRB, Silva MAFS, Oliveira HAABO et al (2022) Platelet-rich fibrin progressive protocol: third generation of blood concentrates. J Oral Maxillofac Surg. https://doi.org/10.1016/j.joms.2022.09.002
Weisel JW, Litvinov RI (2013) Mechanisms of fibrin polymerization and clinical implications. Blood 121(10):1712–1719. https://doi.org/10.1182/blood-2012-09-306639
Ryan EA, Mockros LF, Weisel JW, Lorand L (1999) Structural origins of fibrin clot rheology. Biophys J 77(5):2813–2826. https://doi.org/10.1016/S0006-3495(99)77113-4
Kubesch A, Barbeck M, Al-Maawi S et al (2019) A low-speed centrifugation concept leads to cell accumulation and vascularization of solid platelet-rich fibrin: an experimental study in vivo. Platelets 30(3):329–340. https://doi.org/10.1080/09537104.2018.1445835
Golebiewska EM, Poole AW (2015) Platelet secretion: From haemostasis to wound healing and beyond. Blood Rev 29(3):153–162. https://doi.org/10.1016/j.blre.2014.10.003
Nurden AT (2011) Platelets, inflammation and tissue regeneration. Thromb Haemost 105(Suppl 1):S13–S33. https://doi.org/10.1160/THS10-11-0720
Gawaz M, Vogel S (2013) Platelets in tissue repair: control of apoptosis and interactions with regenerative cells. Blood 122(15):2550–2554. https://doi.org/10.1182/blood-2013-05-468694
Tunalı M, Özdemir H, Küçükodacı Z et al (2014) A novel platelet concentrate: titanium-prepared platelet-rich fibrin. Biomed Res Int 2014:209548. https://doi.org/10.1155/2014/209548
Bhattacharya HS, Gummaluri SS, Astekar M, Gummaluri RK (2020) Novel method of determining the periodontal regenerative capacity of T-PRF and L-PRF: an immunohistochemical study. Dent Med Probl 57(2):137–144. https://doi.org/10.17219/dmp/117721
Tsukioka T, Hiratsuka T, Nakamura M et al (2019) An on-site preparable, novel bone-grafting complex consisting of human platelet-rich fibrin and porous particles made of a recombinant collagen-like protein. J Biomed Mater Res B Appl Biomater 107(5):1420–1430. https://doi.org/10.1002/jbm.b.34234
Acknowledgements
The authors would like to thank Dental Research Center Biomechanics, Biomaterials and Cell Biology (Federal University of Uberlandia) for providing a large part of the structure used in the research.
Funding
This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES/Brazil)-Finance Code 001-nd Fundação de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG/Brazil) for the granting of scholarship and resource for the purchase of consumer material.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by MAFSS, CRBL, PHJOL, MDMAC, HAABO, and PD. The first draft of the manuscript was written by MAFSS, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical Approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Federal University of Uberlandia (No. 13857519.0.0000.5152).
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Silva, M.A.F.S., Linhares, C.R.B., Saboia-Dantas, C.J. et al. Fibrin Network and Platelets Densities in Platelet-Rich Fibrin (PRF) Membranes Produced from Plastic Tubes Without Additives: A New Approach to PRF Clinical Use. J. Maxillofac. Oral Surg. (2024). https://doi.org/10.1007/s12663-023-02103-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12663-023-02103-2