Abstract
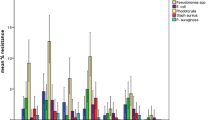
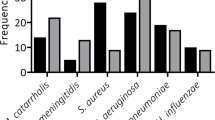
Postoperative infection and sepsis remain a major cause of morbidity among patients undergoing surgery. Maintenance of strict asepsis is essential if post-operative infection and its consequences are to be minimized. In developing countries maintenance of asepsis in most operation theatres is limited to fumigation and mopping. Clinical trials have confirmed that 80–90 % of bacterial contaminants found in wound after surgery come from microbes present in air of operating theatre. A study was conducted to evaluate the microbiological contamination of maxillofacial operation theatres in India and its correlation to weekly fumigation. A total of 6,723 culture plates, including 2,241 air and 4,482 swab samples were studied. Samples were collected at prefumigation, midcycle and post-fumigation stages and were cultured over three different medias. Predominant bacteria identified were Staphylococcus aureus (76 out of 83 samples by active air sampling) followed by Streptococcus while Aspergillus was the main fungal isolate. Formaldehyde based fumigation decreased the colony forming units (cfu/m3) of all organisms in different samples and was found to be highly effective against Fungi and E. coli. Literature suggests that for conventional operating theatres the bioload should not exceed 35 cfu/m3 in an empty theatre. In our study the cfu levels were always lower immediately after fumigation was carried out; however they moved up beyond this limit as the days passed. This implies that once a week fumigation with formaldehyde is less than optimal to achieve acceptable levels of disinfection.


Similar content being viewed by others
References
Smith C (1975) Hospital operating theatre environment and the assessment of filters for use in associated ventilation plants. Ann Occup Hyg 17:303–314
Ajaz M, Qadri GJ, Tabish SA et al (2004) Incidence of nosocomial infection in postoperative patients at a teaching hospital at Kashmir. JK Pract 11:38–40
Meers PD, Ayliffe GAJ, Emmerson AM et al (1981) Report on the national survey of infection in hospitals, 1980. J Hosp Infect 2(supplement):29–34
Raymond DP, Pelletier SJ, Crabtree TD, Schulman AM, Pruett TL, Sawyer RG (2001) Surgical infection and the aging population. Am Surg 67(9):827–832
Kaur N, Hans C (2007) Air bacterial isolations from operation theatres in a tertiary care hospital in India. J Clin Diagn Res 1(2):87–89
Rao TV, Chithra VN (2008) Surveillance and sterilisation of operation theatres in the developing world. http://www.articlesbase.com/medicinearticles/surveillance-sterilization-anddisinfection-of-operation-theatres-inthe-developing-world-327599.html. Accessed 4 Nov 2012
Rautemaa R, Nordberg A, Wuolijoki-Saaristo K, Meurman JH (2006) Bacterial aerosols in dental practice—a potential hospital infection problem? J Hosp Infect 64:76–81
Centers for Disease Control and Prevention. Guideline for prevention of surgical site infection (1999) Infect Control Hosp Epidemiol 20:247–278
Pasquarella C, Pitzurra O, Savino A (2000) The index of microbial air contamination. J Hosp Infect 46:241–256
Michla Y, Holliday M, Geuld K, Weir DJ, McCaskie AW (2006) The impact on bacterial contamination of hip arthroplasty wounds of personal protection helmet system. J Bone Joint Surg Br 88B(III):412
Howarth FH (1985) Prevention of airborne infection during surgery. Lancet 1:386–388
Javed I, Hafeez R, Zubair M, Anwar MS, Tayyib M, Husnain S (2008) Microbiological surveillance of operation theatres and ICUs of a tertiary care hospital, Lahore. Biomedica 24:99–102
Landrin A, Bissery A, Kac G (2005) Monitoring air sampling in operation theatres: can particle counting replace microbiological sampling. J Hosp Infect 61:27–29
Kelkar U, Kelkar S, Bal AM, Kulkarni S, Kulkarni S (2003) Microbiological evaluation of various parameters in ophthalmic operation rooms. The need to establish guidelines. Indian J Ophthalmol 51:171–176
Hoffman PN, Williams J, Stacey A, et al (2002) Microbiological commissioning and monitoring of operation theatre suites. J Hosp Infect 52:1–28
Dharan S, Pittet D (2002) Environmental controls in operating theatres. J Hosp Infect 51:79–84
Grand H (2009) Victorian infection control professionals association biennial state conference 2009
Nakhla LS, Cummings RF (1981) A comparative evaluation of a new centrifugal air sampler (RCS) with a slit air sampler (SAS) in hospital environment. J Hosp Infect 2:261–266
Osaro EF, Ufuoma IO, Dorcas AO (2008) Hospital indoor airborne microflora in private and government owned hospitals in Benin City, Nigeria. World J Med Sci 3:34–38
Prescott LM, Harley JP, Klein DA (2005) Microbiology, 6th edn. McGraw Hill Co., New York
Kelkar U, Bal AM, Kulkarni S (2005) Fungal contamination of air conditioning units in operating theatres in India. J Hosp Infect 60:81–84
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bali, R., Sharma, P., Nagrath, S. et al. Microbial Isolations from Maxillofacial Operation Theatre and its Correlation to Fumigation in a Teaching Hospital in India. J. Maxillofac. Oral Surg. 13, 128–132 (2014). https://doi.org/10.1007/s12663-012-0458-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12663-012-0458-3