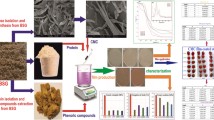
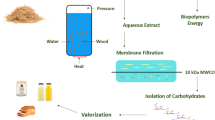
Abstract
Waste composition range from simple sugars to complex polysaccharides such as starch, cellulose, and hemicellulose, including more complex sources such as lignin, lipids, and proteins. This composition suggests its use as raw materials that can potentially be valued by different techniques and used as resources for new food additives, leading to economic and environmental benefits. In this work, the extraction and production of microfibrillated cellulose (MFC) as the way forward to valorise agroindustrial residues. The high surface area and aspect ratio, rheological behaviour, water absorption and absence of cytotoxic and genotoxic properties of MFC studied in the present study facilitate its use in food applications as stabilizing and texturizer agent. This work has two main goals: The first goal is the production of MFC using a method based on the combination of green chemical and mechanical pre-treatment of the feedstock followed by a disintegration process of the cellulose obtained from agroindustrial residues. The results revealed that the different MFC obtained have a quality comparable with commercial MFC, which is reported by the determination of the Quality Index (QI). QI values between 50 and 63 are obtained with the agroindustrial subproducts tested. The MFC was also characterized using several techniques such as FTIR, SEM and TGA. The second goal is the MFC validation as thickener agent in food (mayonnaise, peach nectar, strawberry syrup and puree apple). First, a rheological study was carried out to observe the MFC thixotropic behaviour. Second, to evaluate techno-functional properties in-vivo, MFC was compared in terms of functionality with the seven additives commonly used in the industry: tare gum, xanthan gum, guar gum, carrageenan, pectin, agar and gelatine. The results revealed that the sample with 0.1% MFC exhibited a reduced thixotropic behavior compared to the other samples (1, 1.5 and 2%). Compared with commercial gums, MFC exhibits the highest water activity (0.87 ± 0.00) and the most alkaline pH value (10.10 ± 0.01). In the in vivo dietary models, thermal stabilization values after 15 min at 85 °C indicate that gums perform better than MFC and carrageenan in mayonnaise stabilization. This study also reveals that gums are more effective in stabilizing the cloud, while gelatine or MFC-containing samples exhibit a higher cloud volume after 30 min of stabilization. Notably, the cloud suspension of peach nectar with MFC-containing samples compares favorably to the control without gums (20 to 40%). This comprehensive study underscores the potential of MFC as a versatile and eco-friendly alternative in food applications, aligning with the broader goals of sustainable waste management and environmentally conscious practices in the agroindustrial sector.
Graphical Abstract

Statement of Novelty
The novelty of this manuscript is in terms of the utilization of underestimated agroindustrial wastes namely, cereal husks for the production of microfibrillated cellulose (MFC) via a low cost green process. This shows the projected low cost of such a process on a large scale. The optimization of chemical consumption, energy costs, grinding parameters and the investigation of their effects on the final characteristics add to the distinctiveness of the study. Together with the evaluation as food texturizers. This particular comparison of cellulose microfibers with commercial food texturizers has not been explored so far; our research group has explored and assessed their suitability to be used as a food additive material. This study can thus benefit developing countries, particularly farmers and/or food producers who can derive a secondary source of income from agroindustrial wastes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The increase in agricultural and food residues has become a major concern throughout the world. These materials possess varied compositions (water, minerals, carbohydrates, and lignin contents) depending on the crop source [1]. The residues derived from industrial processing and forest activities should be also included under this composition [2, 3].
Now, the main uses of the agricultural and forestry subproducts are low value-added applications, mainly meeting needs in farming activities (bed and feed for animals), soil fertilization and composting or energetic requirements [4]. These applications do not cover the real potential of these raw materials from a sustainable, technological, and profitable point of view. Considering this fact, these lignocellulosic residues should be considered an optimal sustainable and renewable source of cellulose.
The major components of lignocellulosic biomass are cellulose, hemicellulose, and lignin. The content of these components in biomass varies significantly depending on the type of biomass selected. Woody plants have tightly bound fibers with higher lignin content while annual plants have looser bound fibers, a fact that indicates lower lignin content. Usually, cellulose, hemicellulose and lignin constitute 40–50 wt%, 20–35 wt% and 10–25 wt% of the plant material respectively [5]. Cellulose is the most abundant polymer on earth and its proper utilization promise to contribute to move forward to circular economy. Cellulose has also the advantages of being biodegradable, durable, non-toxic, and thermally and mechanically stable [6].
Cereals are one of the most significant food sources and crops in the human diet. Cereal processing chain generates huge amounts of agricultural waste, which is known as lignocellulosic biomass. It is estimated that 12.9% of all food wastes are produced during cereal processing and manufacturing, and 30% of the cereal weight basis is lost or wasted [7].
New strategies to reuse these value-added products are necessary to reduce the environmental burden [8]. These feedstocks suppose an important source of lignocellulosic biomass ready to be used to produce microfibrillated cellulose (MFC). The present study is focused on MFC production from agroindustrial residues through the combination of chemical and mechanical treatments and the study of its application as food additive, leading to an agroindustrial waste environmental impact mitigation and moving towards a biorefinery concept. The partial (or even total) use of industrial and agricultural wastes not only is an advantage from an environmental point of view, but it could also solve some economic issues [9]. Indeed, this leads to the minimization of residues by means of waste treatment or management and to the development of high value-added products that can provide large profits. MFC has potential use as stabilizing agent in food emulsion. The high surface area and aspect ratio, rheological behavior, water absorption and absence of cytotoxic and genotoxic properties of MFC facilitate its use in food applications [10].
Hydrocolloids are a diverse group of long-chain polymers that are readily dispersive, fully or partially soluble, and prone to swell in water. Hydrocolloids are extracted from common or unique natural sources, including cereal grains, wounded trees, seaweed, animal skin and bones, and fermentation slime. These compounds are usually used as ingredients in the food industry, changing the physical properties of the solution to form gels, or enable thickening, emulsification, coating, and stabilization [11]. The importance of hydrophilic colloids lies in their hydrophilic nature, making important textural components of most foods. Hydrocolloids are employed for an extensive array of applications, used as food additives imparting textural and functional properties. Each type of hydrocolloid has specific characteristics, and these may vary depending on the type of food product for which they are intended. A few examples are clouding agents in fruit juices and nectars; foam stabilizers in whipped toppings and beer; gelling agents in puddings, desserts, aspics, and mousses; protective colloids in flavor emulsions; stabilizers in beer and mayonnaise; and thickening agents in jams, pie fillings, sauces, syrups and gravies. It is important to note that the broad applications of hydrocolloids are confined to their two main properties—to serve as thickening and gelling agents. In addition, they have been used as gelling or thickening agents capable of binding water molecules, thereby enhancing the desired textural properties of foodstuffs [12]. For this reason, hydrocolloids are important in maintaining the desired texture of food products; enhance their self- life and various other physicochemical properties of food [13].
In this current study, we assessed the techno-functional properties of microfibrillated cellulose (MFC) with the aim of employing it as a texturizer across various food matrices. Firstly, the manuscript distinguishes itself by utilizing cereal by-products for the economically friendly creation of MFC, underscoring its potential cost-effectiveness on a large scale. The study’s distinctiveness is further underscored by optimizing chemical consumption, energy expenditures, and grinding parameters, investigating their impact on final characteristics. Secondly, a rheological study was conducted to observe thixotropic behavior and flow characteristics, with the objective of comprehending potential applications of this novel ingredient in formulating new foods. The functional properties of MFC were assessed in various food formulations, such as mayonnaise, peach nectar, edible foams, strawberry syrup, and apple gelatin, formulated with seven additives commonly used in the industry in terms of functionality.
Materials and Methods
Materials
Agroindustrial subproducts provided by Federació de Cooperatives Agràries de Catalunya (FCAC), who works with local agriculture farmers, were used in this work. The reagents used in this work were: acetic acid (ACS reagent, C 99.7%); acetone (Sigma Aldrich); Hydrochloric acid (Sigma Aldrich, 37%), Sodium chloride (Sigma Aldrich [99%); Sodium hydroxide (Sigma Aldrich, C 97%); Sodium Chlorite (Sigma Aldrich, [80%). For in-vivo trials, white sugar (Mercadona, Spain), salt (Mercadona, Spain), Carrefour apple vinegar (Carrefour, Spain), Borgesol sunflower oil (Borges Branded Foods, Spain), pasteurized whole liquid egg (Pascual, Spain), peaches (Prunus persica L.) (Carrefour, Spain), strawberry (Fragaria vesca) (Carrefour, Spain), apple (Malus domestica) of the Royal Gala variety (Carrefour, Spain), Ovopack egg white (Carrefour, Spain) and food gelatin in leaves Vahiné (Carrefour, Spain) were purchased from different supermarkets of Lleida; the additive gum tara (GT), xanthan gum (GX), guar gum (GG), nappage apple pectin (PE), agar (AG) and instant gelatin (GEL) were obtained from Dayelet (Barcelona, Spain). Iota carrageenan (IC) was obtained from Molecule R (Montreal, Canada). Microfibers from cellulose (MC) provided by ITENE. Citric acid, alginate, glucose, calcium chloride, soda and phenolphthalein were purchased from Sigma (Barcelona, Spain).
Equipment
Homogenizer ULTRA-TURRAX T-25 (IKA, Staufen, Germany), Centrifuge Sigma-3-18 KS (Sigma Laborzemtrifugen GmbH, Germany), texturometer TA.XT2 (Stable Micro Systems Ltd., Surrey, England), Minolta CR-200 colorimeter (Minolta INC, Tokyo, Japan), pH-meter Basic 20 (Crison Instruments S.A., Spain), water activity mediator Aqualab (Decagon Devices, Spain), Gram Precision Serie SV analytical balance (Barcelona, Spain), blender handheld MQ100 (Braun, Spain), refractometer PAL-BX ACID 5 (Atago, Tokyo, Japan) and Haake RheoStress 600 rheometer (Bacchus Marsh, Victoria, Australia).
Feedstock Characterization
The initial characterization of the agroindustrial subproducts was conducted according to their chemical composition. The following TAPPI standard methods (Technical Association of the Pulp and Paper Industry) were used for this purpose: moisture (Tappi standard, T264 cm-97), inorganic content (Tappi standard, T211 om-93), Klason lignin (Tappi standard, T222 om-98), holocellulose (Wise et al. method), cellulose (Rowel method) and hemicelluloses. The difference between holocellulose and cellulose were quantified for evaluating the initial potential of these waste streams for the aim of this work.
Microfibrillated Cellulose Extraction from Agroindustrial Wastes
The isolation of MFC from agroindustrial subproducts was achieved through the application of a combination of chemical and mechanical treatments. The phases of the whole process were chosen based on the approach of developing an environmentally friendly process for the obtaining of biomaterials from this agricultural waste stream [14]. The processes developed for the isolation of cellulose are based on alkaline pre-treatments applied in the pulping industry. For the cooking stage, soda cooking process was accomplished as a sulphur-free delignification method. First, the feedstock was cooked with NaOH. The suspension was heated until 140 oC with continuous stirring into a digester (DBLP Digester, IDM Test, Spain). This process removed high percentage of the lignin and the hemicellulose fraction.
Then, the solid residue obtained in the previous stage was loaded in the digester and adjusted with water to a consistency of 10%. After, reagents were added in the appropriate proportion: H2O2 (4%), NaOH (3%), Na2SiO3 (2.5%) and DTPA (0.3%). Next, the vessel was closed, and the mixture was heated to 70 °C during 120 min with continuous stirring, in order to remove the residual lignin. The selected process for this purpose was a total Chlorine Free Bleaching.
The bleached cellulose was disintegrated to reduce the size of the fibers up to micrometric size. The disintegration process was carried out with a grinder (Super Mass colloider, Masuko Sangyo, Japan) with two specific stones; a static and a rotating grinding stone, this combination facilitates the breakdown of the cell-wall structure of the fibers [15]. The cellulose was fed into the grinder and subjected to several passes between the stone section, which was set to 1500 rpm, until a gel consistency is obtained. Gel consistency is achieved with a 50,000 Cps viscosity at 10 rpm determined with a viscosimeter (Brookfield DV-E, AMETEK Brookfield, EE. UU.).
Microfibrillated Cellulose Characterization
To evaluate the quality of the distinct types of MFC, the Simplified Quality Index (SQI), reported by Desmaisons et al. [16], was employed. Through the application of the quality index, the different samples of MFC can be compared easily due the results obtained in four different assays summarized in a unique punctuation, avoiding the need of comparing the results obtained test by test. In contrast with the original quality index, the simplified version is based on four parameters (nanosize fraction, turbidity, area of microparticles and Young’s modulus), which are pondered and used for the SQI calculation according to the following equation:
where x1 is the nanosized fraction (%), x2 is the turbidity (NTU), x3 is Young’s modulus (GPa) and x7 is the microparticle area (mm2).
Nanosize fraction: 0.02% MFC suspensions were treated in a centrifuge (Thermo-scientific Sorvall RC-6, EE. UU.) at 2700 rpm for 15 min and, next, the supernatant was dried at 105 °C. The relation between the concentration of fibers in the supernatant and the initial concentration of the suspension indicates the amount of material comprised in the nano-scale range.
Turbidity: the scattered light (860 nm at 90°) of 0.1% MFC suspensions were measured using a portable turbidimeter (Lovibond TB 300 IR, Germany).
Young’s Modulus: nanopapers obtained using a Rapid Köthen sheet former (IDM Test, Spain) were conditioned for 48 h at 23 °C and 50% humidity. Next, they were cut (10 cm long, 15 mm wide) and subjected to a tensile test using a universal Testometric machine (M350-20CT, England), with a test speed of 10 mm/min.
Area of microparticles: images of the 0.1% MFC suspensions were acquired using an optical microscopy (Leica DM3000, Germany), equipped with a digital camera. Next, photographs were analyzed with the ImageJ software with the purpose of obtaining the required parameter.
The data collected with these parameters provide comparatives values to validate the alternative sources of cellulose selected for this study compared with commercial MFC.
Infrared Spectroscopy Analysis
The chemical structure of the MFC obtained with each agroindustrial subproducts was evaluated by Fourier Transformed Infrared Spectrophotometer (FTIR). FTIR Tensor 27 (Bruker) with ATR Iens (Specac). The tests are performed in duplicate. ATR FT-IR spectroscopy was conducted over the wavenumber range 650–4000 cm− 1. 16 scans were taken for each spectrum at a resolution of 4 cm− 1. Analysis was performed on MFC dried by spray drier.
Thermogravimetric Analysis (TGA)
The thermal stability of produced MFC were studied via TGA and then compared with commercial MFC. 10 mg was utilized for thermogravimetric analysis (TGA) and derivative thermogravimetry (DTG) using a TGA Q5000 IR (TA Instruments), where the samples were heated to 900 ºC under nitrogen atmosphere at room temperature with a heating rate of 20 ºC/min.
Scanning Electron Microscopy (SEM)
Scanning electron microscopy (SEM) photographs of MFC were captured using Hitachi SEM S4100 under accelerated electrons with 10 kV of energy. Before the morphological evaluation by SEM, the samples were freeze-drying and coated with Au-Pd using the sputtering technique.
Rheological Properties of MFC
Rheological parameters, including thixotropy and flow curve of the advanced cellulose fibers (MFC), were determined following the procedures described by Ibarz et al. (2010). Four different concentrations, including 0.1, 1.0, 1.5 and 2.0 wt % of MFC were used. Each sample was mixed with distilled water (300 mL) using the Ultra Turrax T-25 (IKA, Staufen, Germany) during 2 min at 16,000 rpm. The rheological characterization was assessed using Haake RheoStress 600 rheometer (Bacchus Marsh, Victoria, Australia) with a Haake Z40-DIN concentric cylinder.
The rheological behavior of food fluids can be described by XXXifferrent constitutive equations that correlate the two basic measures of thixotropy and flow behavior.
To quantify the thixotropic behavior, the experimental data were fitted to the Figon-Shoemaker (1983) Eq. (1). In this case, only the sample with 0.1% MFC was characterized at 5, 15, 30 and 45 °C with a shearing force of 80 s− 1 during 300 s.
where: σ is the shear stress (Pa), σ1 and σe are the initial and equilibrium stresses (Pa), which are related with initial and long times, respectively, t time (s) and k is a breakdown kinetic constant.
The flow behavior was fitted using the Newtonian fluid model, (Eq. 2). Rheological measurements were performed in the shearing force range from 0.1 to 80 s− 1 for different temperatures (5, 15, 30 and 45 °C), and at different concentrations (0.1, 1.0, 1.5, 2.0%). Previously, the thixotropic behavior of the samples was eliminated.
where: σ is the shear stress (Pa), η viscosity (Pa) and ɣ̇ shear rate (s−1).
Physicochemical Analysis of MFC and Reference Ingredients
The physicochemical characteristics of MFC were determined and compared to those of commercial additives: tara gum (E-417), xanthan gum (E-415), guar gum (E-412), apple pectin (E-440a), agar (E-406), gelatin (E-428) and carrageenan (E-407). The aw of the MFC and commercial additives were determined with AquaLab (Decagon Devices Inc., Pullman, USA). Total soluble solid was expressed as °Brix and measured using hand refractometer (PAL-X ACID 5 (Atago, Tokyo, Japan)). The pH was assessed with the Basic 20 electronic pH meter (Crison Instruments S.A., Barcelona, Spain). The acidity was determined by diluting 10 mL of the sample with 10 mL of distilled water and titrated with 0.1 N NaOH until pH 8.2 was reached. Results were expressed as mg of malic acid per L. Color was measured using a Hunter Lab colorimeter CR-200 Minolta (Minolta INC, Tokyo, Japan) with an illuminance value of D65/10 equipped with a CR-400. Colour was expressed in terms of L* (darkness/whiteness), a* (greenness/redness), and b* (blueness/yellowness). The chroma (Ch) was calculated using Eq. (3) [17].
Techno-Functional Properties in Vivo Food Models
In order to evaluate techno-functional properties in vivo, the MFC were compared in terms of functionality with seven additives commonly used in the industry, namely tare gum (E-417), xanthan gum (E-415), guar gum (E-412), carrageenan (E-407), pectin (E-440a), agar (E-406) and gelatin (E-428). The different ingredients were evaluated in different food models including mayonnaise, peach nectar, edible foams, strawberry syrup and apple gelatin. Concentrations of each additive were determined in preliminary studies. All the results were compared with a control sample (CK) without the addition of additive. Colour differences (ΔE) were calculated according to Eq. 4 considering as reference (Control, Ct) [11].
Texture properties of all formulated products were measured with TA.XT2 texturometer (Stable Micro Systems Ltd., Surrey, England) connected to Exponent v. 5.0.6.0 software, using a 20 mm (P/20) diameter of cylindrical probe. The measurements were carried out at room temperature (24 ± 1 °C). The extension force-displacement curve of mayonnaise, strawberry syrup and apple gelatin was obtained. From this, firmness (N), consistency (N·s), and rupture strength (N·s) were all obtained using the analysis of the force versus time curve for a two-cycle compression, with a disk probe at 5 mm of distance, with pretest speed of 1 mm/s, test speed of 2 mm/s, and posttest speed of 10 mm/s. Fragility (mm) and adhesiveness (N·s) of strawberry syrup were also performed using TA.XT2 texturometer. The sample was placed at the center of the probe in a flat form on the sample table. A two-cycle compression was measured, with a disk probe (of 35 mm diameter) with pretest speed of 3 mm/s, test speed of 2 mm/s, and posttest speed of 10 mm/s. In-built software of the texture analyzer was used for analyzing the data generated. This process was carried out in triplicate.
Emulsifying Capacity of Mayonnaise
Formulation for making mayonnaise was processed according to Huang et al. (2016) with some modifications. Each mayonnaise sample contained 2.3% sugar, 1.2% salt, 7.1% vinegar, 71.4% sunflower oil, 17.9% egg and 0.1% of the studied additive. Sugar, salt, vinegar, egg and additive were weighted and mixed with ultraTurrax T-25 homogenizer (IKA, Staufen, Germany) for 2 min at 20.000 rpm while adding the sunflower oil drop by drop.
All the samples were placed in glass containers with the objective to achieve the same volume of mayonnaise. Experiment was repeated three times with three replications per treatment. Finally, the thermal stability of the mayonnaise emulsion was calculated. Mayonnaise samples were left in a water bath at 85 °C for 15 min and centrifuged at 4 °C for 10 min at 14,600 rpm. Once centrifuged, the residual water and oil were removed from the mayonnaise following the method of Lafarga et al. [18]. Finally, the samples were weighed with the objective to calculate the percentage of thermal stability (TE) of the emulsion through the following equation:
Where: Pf was the weight of the mayonnaise samples after the thermal stabilization; and P0 was the initial weight of the mayonnaise samples.
Stabilizing Potential in Peach Nectar
Peach nectar was prepared with 30% of fresh peach and 70% of distilled water. The addition of the other ingredients was performed following the method of Coelho et al. [19], with 11% of soluble solids (° Brix) and 0.1% of each of the studied additive. Peaches were peeled, cut and crushed with distilled water using the hand blender for 2 min. Then, sugar was added to adjust the °Brix to reach 11%. The mix was placed in a 2 L beaker. Since nine different samples had to be prepared, additives were added and homogenized with the Ultra Turrax T-25 (IKA, Staufen, Germany) for 2 min. The foam formed was removed and 50 mL of each sample was placed in three tubes, in order to have the sample in triplicate. Finally, samples were stored in the refrigerator at 4 °C and the stabilizing power of the additives was measured by applying an adapted version of the methodology described by Babbar et al. [20], calculating the percentage of suspension (cloud) in the peach nectar. Cloud volume was measured every 30 min and every 60 min until stabilization. Afterwards, the samples were kept and analyzed under refrigeration for 24 and 168 h.
Foaming Capacity and Foam Stability
For the preparation of foam with the different hydrocolloids selected, 100 mL of egg white and 0.1 g of the studied additive. The samples were weighed (20 mL in triplicate) and placed in beakers. Samples were homogenized with Ultraturrax T-25 (IKA, Staufen, Germany) for 50 s at 16,000 rpm, analyzing the foam volume every 10 min. After 30 min, the readings were performed at 30 min-intervals.
Thickening and Viscosity Capacity in Strawberry Syrup
Strawberry syrup was prepared with 10% of strawberry pulp, 60% of water, 30% of sugar, 0.05% of citric acid and 0.1% of of the studied additive. The homogenate was concentrated at 180 °C, until it reached 60 °Brix, following the method described by Coelho et al. [19]. The samples were stored at 4 °C for 24 h. The parameters measured were the gel strength, rupture force, brittleness/elasticity and adhesiveness of a marmalade formulation using the texturometer TA.XT2 (Stable Micro Systems Ltd., Surrey, England) connected to Exponent v. 5.0.6.0. software as described above.
Gelling Capacity in Apple Gelatin
For the preparation of the apple gelatin, 65% apple pulp and 35% of water were weighed (200 mL) in triplicate. Samples were heated at 65 °C. In parallel, 1% alginate, 2% gelatin sheets, 3.5% of the studied HC, 35% sucrose, 10% glucose, 0.2% calcium chloride were weighed and added to apple samples, following the method described in Coelho et al. [19]. Homogenates were arranged in petri dishes of 50 mm diameter and 10 mm height and stored in the refrigerator during 24 h prior to analysis. The texture of apple structures was measured as described above.
Statistical Analysis
Results are expressed by mean ± standard deviation (SD) of 3 repetitions. All data were checked for significant differences by applying analysis of variance test (ANOVA). The criterion for statistical significance was p < 0.05. When significant differences were observed, Tukey’s Honest Significant Difference (HSD) of the means was applied. All statistical analysis was carried out using JMP 13 (SAS Institute Inc., Cary, USA).
Results
Feedstock Characterization
The chemical composition according to their macromolecular composition of used raw materials is shown in Table 1:
The percentage of ashes determined is adequate in all the agroindustrial subproducts analyzed, with wheat bran residues showing the highest content. A high percentage could be problematic for the cooking process, since it could cause corrosion problems in the equipment.
The percentage of holocellulose, understood as the total amount of polymeric carbohydrates (cellulose and hemicellulose), constitutes around 50% in most of the by-products analyzed. As might be expected, wheat bran (wood raw material) has the highest percentage of holocellulose (57%). On the contrary, the potato fiber has the lowest cellulose content.
On the other hand, and as expected given the nature of the waste, the lignin content is low and very similar in the analyzed by-products. These low lignin contents will facilitate the cellulose extraction process, which can be carried out through less aggressive and cheaper treatments, since the amount of lignin to be separated from the cellulose is less. Wheat bran and potato fiber present lower lignin content, constituting a less recalcitrant feedstock.
Based on the conclusions drawn from the analysis of the chemical composition of the evaluated by-products, similar yields could be expected. Considering these compositions, raw materials analyzed are suitable biomass sources to obtain cellulose and microfibrillated cellulose.
Microfibrillated Cellulose Extraction from Agroindustrial Wastes
The yield reached in each stage will be calculated taking into account the amount of material (solid content) at the beginning and at the end of each stage Table 2.
The yield in the cooking process is the lowest of the whole process due, in this stage, a great part of the compounds of the feedstock are solubilized in the black liquor. In the bleaching process, colored compounds are removed, so there is also a loss in the weight of the product. Yields of 50% and 66% for pulping and hydrogen peroxide bleaching, respectively, are reported for pine wood raw material [21]. In other study with coconut husk fibers yields of 50 and 22% for pulping and bleaching respectively were obtained [22]. Therefore, yields attained with the raw materials evaluated were promising. Finally, the yield in the last stage (mechanical treatment) is very high due to no compounds are eliminated.
Microfibrillated Cellulose Characterization
The comparison of the MFC obtained from each agroindustrial subproduct was carried out by obtaining the Simplified Quality Index. The results obtained in each trial are detailed in Table 3. Commercial MFC reference was also characterized for comparative purposes.
According to the results, oat husk has obtained the highest punctuation (61.6), although the quality index of wheat bran and beer bagasse are quite similar (60.7 and 59.0, respectively), which implies comparable qualities. The quality offered by potato fiber value is clearly the lowest (34.2). Regarding spelt, the punctuation obtained indicates a medium quality. The use of agroindustrial subproducts make possible to obtain MFC with qualities similar or higher that hardwood kraft pulp MFC with different chemicals pretreatments (QI between 55 and 70) [21].
Based on these results, oat husk was the subproduct selected to be used as texturizers in different food matrices. In addition, a commercial grade available was selected as a benchmark to validate the use of the agroindustrial subproducts as alternative sources for the production of MFC. Moreover, all subproducts evaluated are suitable as MFC feedstock and comparable with commercial benchmark, except for the oat husk, which presents a QI much lower than commercial [22]. Some of the agroindustrial subproducts evaluated present values comparable to those of the literature produced from eucalyptus fibres and enzymatic pre-treatment.
Infrared Spectroscopy Analysis
Figure 1 shows the FTIR spectrum for the different MFC produced. 3000 and 2845 cm− 1 peaks correspond to O-H and C-H stretching vibration of CH2-OH groups of cellulose structure. Despite analyzing the dried samples, the peak detected at 1743 cm-1 in beer bagasse MFC is due to O-H bending of absorbed water during the test. Any peak detected at 1510 cm-1 related to the aromatic ring vibrations of lignin indicates no residual lignin content. The peaks at 1370, 1162, and 1110 cm− 1 correspond to C-H vibrations, glycosidic bonds (C–O–C) and C–OH, respectively. The intense peak at 1600 cm-1 is due to C–C stretching related with the cellulose structure.
Thermogravimetric Analysis (TGA)
Figure 2 shows the TGA curves and their first derivates, which give the mass loss as a function of the temperature, of control commercial MFC reference is included. It was possible to observe one main degradation process in all references. The first mass loss was observed before 100ºC and is associated with water evaporation. The main degradation stage was found between 350 and 360 ºC with a mass loss of about 75% and was attributed to the pyrolysis of cellulose fibers [23]. An increase in the decomposition temperature represents higher thermal stability which was higher in Oat husk MFC (363ºC). These values were in good accordance with the commercial MFC reference.
Scanning Electron Microscopy of MFC
The evaluation of the morphology and the size of the MFCs were carried out by SEM. The following figures show SEM images of the disintegrated cellulose fibers at 50kX and 60kX magnifications.
The SEM morphology of the different MFC evaluated are shown in Fig. 3. They display a classical morphology. MFC isolated from potato fibers (Fig. 3d) present different morphology with diameters of 30–40 nm. The different cereal husk evaluated show a uniform aspect with diameters of 40–50 nm.
Rheological Characterization of MFC
Many hydrocolloids are selected for enhancement of functional properties, such as water holding capacity and rheological properties. The rheological properties are influenced by the type and concentration of ingredients. However, they are useful in industrial applications because they are effective at low concentrations. For this reason, in the present study, the rheological characterization was only determined in cellulose microfibers (MFC) at 0.1, 1.0, 1.5 and 2.0% in distilled water (v/v).
Thixotropy
For a constant shear force of 80 s− 1, the variation of the shear stress with time can be observed in Fig. 4. With the different concentrations (0.1, 1.0, 1.5 and 2.0%) of MFC, the equilibrium of the shear stress was reached at 18 s, with no viscosity change during time. These results showed that there was no thixotropy in the samples studied, since this parameter disappeared rapidly (≈ 18 s).
Values of the rheological parameters of the Figoni-Shoemaker model as a function of the percentage of MFC at 80 s− 1 of shear force are represented in Table 4. The samples with 0.1% and 1.0% MFC presented the lowest σe value (0.139 Pa), which indicates lower viscosity compared to 1.5 and 2.0% samples. The σi-σe parameter indicates the amount of structure that has been destroyed during the shear stress, observing that this parameter was higher in the 0.1% samples compared to the other concentrations (1.0, 1.5 and 2.0%) tested. For this reason, 0.1% presented the highest value of the constant k, reaching the equilibrium earlier. It can be concluded that the 0.1% sample has a lower thixotropic behavior than the rest of the samples, being also the easiest to eliminate. This could be due to the fact that the soluble solids and fiber content is lower in the 0.1% formulation [24].
Flow Curve
The flow curve represents the stress of the samples versus the shear force (0.1–80 s− 1). The values of Newtonian viscosity for the different samples and temperatures studied were represented in Table 5. The present results shown that the viscosity (η (mPa·s)) increases with increasing the MFC concentration and decreases with increasing the temperature (5–45 °C). In all cases studied, the rheological behavior of the samples analyzed was Newtonian. The experimental results of the variation of shear stress with shear force for the samples studied at 5 °C were represented in Fig. 5.
In summary, all the samples tend to a constant straight line intercepting the origin, behaving like a Newtonian fluid [25]. Furthermore, where the higher the concentration, the greater the viscosity of the sample was. This fact could be explained due to the straight line with the higher the concentration, present the greater pendent (viscosity). In conclusion, the addition of MFC did not affect the rheological behavior of the samples. The rheograms obtained for the rest of temperatures present similar flow behaviors (data not shown).
Physicochemical Analysis of MFC and Reference Ingredients
The water activity (aw) did not show any significant (P > 0.05) difference among the varied hydrocolloids studied (Table 6). The maximum aw was noticed in MFC (0.87 ± 0.00) and the xanthan gum showed the minimum moisture content (0.27 ± 0.00) [26]. The value of pH significantly varied between 4.58 ± 0.04 and 10.10 ± 0.01 (P < 0.05). The highest pH value (10.10 ± 0.01) was observed in MFC and the lowest pH value (4.58 ± 0.04) was observed with pectin.
The colour of the different hydrocolloids studied can be described as yellowish-white, being an important parameter with respect to consumer preference. The L*, a* and b* values of the different hydrocolloids are tabulated in Table 7. At same concentration, the different samples tested showed L* values higher than 85%. The b* value showed the yellowness in the ingredients ranged between 7.35 and 13.18, with MFC being the sample with the lowest b* value (P < 0.05).
Techno-Functional Properties in Vivo Food Models
Emulsifier Capacity and Stability of Mayonnaise
Mayonnaise is a semi-solid oil-in-water (O/W) emulsion. One of the main factors that pronouncedly influence the characteristics and stability of the O/W emulsions is the occurrence of polysaccharides in the aqueous continuous phase. Therefore, hydrocolloids are biopolymers of hydrophilic with high molecular weights that possess the properties of thickening, stabilization, the formation of coatings, water retention and gelling, and are commonly used to stabilize the texture of emulsions and to give desirable sensory properties in food products [27]. For the mayonnaise prepared with the different texturizers studied, firmness observed with carrageenan, pectin and MFC did not differ from the control samples without HC (Table 8). Gums obtained the highest values, being guar gum and tara gum the ones that presented the highest firmness values. Amin et al. [28] observed a greater cohesion and gumminess in mayonnaise samples to which guar gums were added and xanthan, due to the increase in viscosity in the emulsion. Regarding penetration, its value correlates positively with the firmness.
The thermal stabilization values of the different mayonnaise elaborated with hydrocolloids are shown in Figure 6. The gums presented the highest thermal stabilization values after 15 min at 85 °C, while MFC together with carrageenan presented the lowest mayonnaise stabilization values (<75 %).
Regarding colour, L*, a* and b* coordinates as well as Ch and δE of mayonnaise were represented in Table 9. All the samples studied, showed a high brightness value (L *> 85), with a yellow hue in all cases with remnants of green pigment. The formulation obtained with the three gums showed the same luminosity value (P < 0.05). These colour differences are related to the types and quantities of some components present in the product [29]. In all formulations, the yellowish coordinate (b*) were equivalent among the different formulations, being the δE value < 3.00, which means that these differences were not visible to the human eye, as we can observe in Fig. 7.
Potential Stabilizer in Peach Nectar
Several hydrocolloids have been widely used in the food industry, aimed to provide the gel structure, increase viscosity, act as encapsulating agent in formation of films, control crystallization, inhibit syneresis, and increase the physical stability of the products. These hydrophilic polymers can directly influence the properties of foods, such as appearance and texture. The attributes colour and turbidity are decisive for the acceptance of juices and nectars, which should not present sedimentation or phase separation, even with preservation of the nutritional value and taste. Phase separation is associated with chemical interactions, density between the disperse phase and dispersant, particle size and viscosity of the disperse phase [30]. In this context, with the objective to extend the stability of nectar cloud, the different hydrocolloids studied were added to peach nectar. The peach nectar cloud is represented in Fig. 8. In the present study, the gums were more effective in stabilizing the cloud, while the samples with gelatin or MFC presented a higher cloud volume after 30 min stabilization.
In Fig. 9A the cloud suspension of peach nectar is represented throughout the time (9 h). During the first 8 h, the stabilization of nectar cloud was higher in xanthan gum and tara gum samples, with 100% of stability. However, MFC-containing sample was comparable to control (CT), with values ranging between 20 and 40%. For the stabilization of nectar cloud after 24 h and 7 days (Fig. 6B and C), no significant differences were observed (P < 0.05) between the two sampling points; only in the case of the tara gum and xanthan gum, where at 24 h the two samples presented 100% of stability, but after 7 days the tara gum decreased to 90%. The remaining samples did not vary their percentage in any case.
Cloud behavior in peach nectar with different hydrocolloids (A) every 30 min until stabilization (B) after 24 h (C) after 7 days. (A) tara gum (B) xanthan gum (C) guar gum (D) carrageenan (E) pectin (F) agar (G) gelatin (H) MFC and (I) control (CT). Small letters indicate significant differences between formulations (p<0.05)
Regarding colour, L*, a* and b* coordinates as well as Ch and δE of peach nectar were represented in Table 10. The highest luminosity (L* values) were documented in xanthan gum, carrageenan and pectin with values > 39.74. As for the δE, all values are higher than 3.00, and, therefore, presenting significant differences in consumer perception with respect to control. This fact, considering the small amount of HC used, could be due to the increased retention of air bubbles during homogenization.
Foaming Capacity and Foam Stability
Besides the raw food material, the stability of the foamed product can be improved by the addition of foaming agents and foam stabilizers as well as interactions between them. As reported in literature, various foaming agents such as whey protein concentrate, soy protein isolate, or egg white were examined to obtain stable foams [31]. In order to achieve high foam stability, foam decay needs to be retarded. One possibility is to increase the viscosity of the continuous aqueous phase. The addition of hydrocolloids, which are generally used as gelling and thickening agents in foods, leads to an increase in bulk viscosity and thus, a reduction of gravitational drainage, providing an additional polymeric network that increases foam stability. Several studies have shown that the formation of electrostatic complexes between pectin and egg white protein [32]. The high viscosity of HCs also prevents the coalescence of bubbles [33]. In Fig. 7A the foam capacity of the different hydrocolloids studied are represented. The hydrocolloid sample with the highest foam capacity was xanthan gum-containing foam compared to CT sample. However, guar gum, tara gum and carrageenan were equivalent to xanthan gum in terms of foam capacity (p > 0.05). MFC was equivalent to CT sample, achieving the worst results. In Fig. 7B the stability of the foam was represented over time (150 min). In the present investigation, a rapid decrease was reported in the xanthan gum, guar gum and carrageenan samples, whose have shown the greater foam capacity in Fig. 10A. Regarding MFC-containing foam, the reported stability was low, which indicates that its use as foaming agent would not be recommended. In general, there was a global trend of stabilization of all samples between 30 and 60 min.
Thickening and Viscosity Capacity in Strawberry Syrup
It is important to note that the broad applications of gums are related to their two main properties—to serve as thickening and gelling agents—. The thickening ability, that is, viscosity production, is the key feature in the use of hydrocolloids as bodying, stabilizing, and emulsifying agents in foods. This thickening capacity is achieved by thickening agents, which can increase the viscosity of a liquid without substantially changing its other characteristic properties. HC are used as thickening agents and improve the suspension of other ingredients or emulsions by increasing the stability of the liquid. These thickening agents are mostly used as food additives and are used for products such syrups, sauces, soups or puddings.
To measure the thickness of the strawberry syrup prepared with different hydrocolloids, the brittleness, chewiness and adhesiveness were studied (Table 11). The highest values of brittleness were observed in xanthan gum, carrageenan, gelatin, agar and CT samples, and the lowest values can be seen in strawberry syrup prepared using MFC, guar gum and pectin. The adhesiveness and chewing values of HC-containing syrup were 0.15 ± 0.01 N and − 0.03 ± 0.44 N·s, respectively, with no significant differences observed among the different strawberry syrups elaborated. Molina-Rubio et al. [34] found that carrageenan was the best choice of the HC studied, modifying the rheological properties of the semi-liquid pastry syrup, which consisted of sucrose, high fructose corn syrup, gum, citric acid and water.
Results of colour of strawberry syrup (L*, a* and b* coordinates, Ch and δE) are represented in Table 12. The samples with the highest brightness (L*) were those containing pectin, tara gum and guar gum (P < 0.05). In all formulations a red-yellow hue was observed, where pectin obtained the highest values of both parameters (P < 0.05). As for Ch, pectin obtained the highest value, followed by MFC and tara gum with equal values (P < 0.05). Finally, δE was higher than 3.00 in all samples tested, except in carrageenan and agar-containing samples. The control (CT) shown the lowest compared to the other samples. Thus, the addition of HC to the strawberry syrup during preparation influences the final colour of the product, except in carrageenan and agar, which it did not affect. Figure 11 shows the photographs of the nine different strawberry syrup formulations.
Gelling Capacity in Apple Gelatin
Hydrocolloids have gelling abilities that are helpful in foods where shape retention is needed before any application of pressure. Gelling agents provide the texture of foods through the formation of a gel. The main ones are pectins, gelatine, agar-agar and carrageenan, which are used in the food industry to produce foods such as jams, jellies or restructured food.
The texture values (firmness and consistency) of the different HC-containing samples are represented in Table 13. The pectin-containing samples showed the highest firmness values compared to the CT sample. In the results of the study by Coelho et al. [19] where prepared structured passion fruit, a greater firmness was observed in passion fruit gelatin formulated with PE compared to the control (without additive) which was much smoother. Firmness of the apple gelatin containing MFC was not significantly different from that of the control. It was observed that with the increase in the firmness parameter in the apple gelatin, the consistency of the samples was also increased. As for consistency, the apple gelatin containing pectin or agar showed the highest tensile strength of 8.30 ± 0.78 and 9.00 ± 0.59 N·s, respectively. The lowest tensile strength was noticed in the MFC-containing samples and CT, with 1.34 ± 0.14 and 1.13 ± 0.03 N·s, respectively. Thus, the addition of MFC did not affect neither the firmness and the consistency of apple gelatin.
Regarding colour, L*, a* and b* coordinates as well as Ch and δE of apple gelatin were represented in Table 14. The samples that presented a higher brightness (L*) value were gelatin and CT (P < 0.05). The highest values in terms of green traces were for the CT samples followed by xanthan gum (P < 0.05). In the case of gelatin, a higher value of b* was observed than in the other formulations (P < 0.05). As for Ch, gelatin obtains the highest value, followed by CT (P < 0.05). Finally, δE presented values greater than 3.00 for all formulations, except in the case of guar gum, therefore there are visible differences in colour compared to CT (Fig. 12).
Conclusion
In this study, the production of microfibrillated cellulose from five agroindustrial byproducts were evaluated. First of all, raw materials were characterized using the TAPPI standards, observing that the percentage of cellulose in the different raw materials was very similar. Next, the MFC was produced through a combination of chemical and mechanical treatment. Finally, the distinct types of MFC were characterized using the simplified quality index. In conclusion, it has been demonstrated that distinct types of agroindustrial byproducts may be valorized to obtain MFC. Quality Index between 50 and 63 were obtained. Additionally, FTIR, TGA and SEM analysis were performed to compare the properties of the different MFC produced.
In the second part of this manuscript, the best performance MFC was characterized in terms of rheological behavior. This MFC was used as a texturizer in several food matrices and compared to commercially available food additives. The highest water activity (aw) was found in Microfibrillated Cellulose (MFC) at 0.87 ± 0.00, accompanied by the highest pH value of 10.10 ± 0.01. Notably, gums demonstrated superior thermal stabilization at 85 °C for 15 min, while MFC, combined with carrageenan, exhibited the lowest stabilization values in mayonnaise (< 75%). Gums were more effective in stabilizing the cloud, while gelatin or MFC-containing samples showed higher cloud volumes after 30 min of stabilization. MFC was also used to stabilize peach nectar and was found to have an effect similar to that of agar, pectin, and carrageenan (20 to 40%). The hydrocolloid sample with the highest foam capacity was the one containing xanthan gum. In terms of adhesiveness and chewing values, the hydrocolloid-containing syrup recorded 0.146 ± 0.01 N and − 0.03 ± 0.44 N·s, respectively, with no significant differences observed among various strawberry syrups developed.
Overall, these findings emphasize the importance of Microfibrillated Cellulose as a texturizing agent in various food products, including mayonnaise, peach nectar, and strawberry syrup, providing valuable insights into their stability, suspension, and sensory attributes. Nevertheless, it is important to highlight that further studies are needed to fully understand their potential for being used as additives in different food products, or in the same food matrices but at different concentrations or temperature and pH conditions.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Roy, P., Mohanty, A.K., Dick, P., Misra, M.: A review on the challenges and choices for food waste valorization: Environmental and economic impacts. ACS Environ. 258–75 (2023). https://doi.org/10.1021/acsenvironau.2c00050
Plakantonaki, S., Roussis, I., Bilalis, D., Priniotakis, G.: Dietary fiber from plant-based food wastes: a comprehensive approach to cereal, fruit, and vegetable waste valorization. Processes 2023, 11, 1580. (2023). https://doi.org/10.3390/pr11051580
Said, Z., Sharma, P., Nhuong, Q., Bora, B., Lichtfouse, E., Khalid, H., Luque, R., Nguyen, X., Hoang, A.: Intelligent approaches for sustainable management and valorisation of food waste. Bioresour. Technol. 377(128952), 0960–8524 (2023). https://doi.org/10.1016/j.biortech.2023.128952
García, A., Gandini, A., Labidi, J., Belgacem, N., Bras, J.: Industrial and crop wastes: A new source for nanocellulose biorefinery. Ind. Crops Prod. 93, 26–38 (2016)
Borrero-López, A.M., Valencia, C., Franco, J.M.: Lignocellulosic materials for the production of biofuels, biochemicals and biomaterials and applications of lignocellulose-based polyurethanes: a review. Polymers, vol. 14, nº 5, p. 881, 2022. (2022)
Salas, C., Nypelö, T., Rodriguez-Abreu, C., Carrillo, C., Rojas, O.J.: Nanocellulose properties and applications in colloids and interfaces. Curr. Opin. Colloid Interface Sci. 19(5), 383–396 (2014)
Fărcaș, A.C., Socaci, S.A., Nemeș, S.A., Salanță, L.C., Chiș, M.S., Pop, C.R., Vodnar, D.C.: Cereal waste valorization through conventional and current extraction techniques—an up-to-date overview. Foods. 11(n– 16), pp24–54 (2022)
Fritsch, C., Staebler, A., Happel, A., Márquez, M.A.C., Aguiló-Aguayo, I., Abadias, M., Gallur, M., Cigognini, I.M., Montanari, A., López, M.J., Suárez-Estrella, F., Brunton, N., Luengo, E., Sisti, L., Ferri, M., Belotti, G.: Processing, valorization and application of bio-waste derived compounds from potato, tomato, olive and cereals: A review. In Sustainability (Switzerland) (Vol. 9, Issue 8). MDPI. (2017). https://doi.org/10.3390/su9081492
Haldar, D., Purkait, M.K.: A review on the environment-friendly emerging techniques for pretreatment of lignocellulosic biomass: Mechanistic insight and advancements. In Chemosphere (Vol. 264). Elsevier Ltd. (2021). https://doi.org/10.1016/j.chemosphere.2020.128523
Perumal, A.B., Nambiar, R.B., Moses, J.A., Anandharamakrishnan, C.: Nanocellulose: Recent trends and applications in the food industry. Food Hydrocoll. 127, 107484 (2022)
Williams, P.A., Phillips, G.O.: Introduction to food hydrocolloids. Handbook of Hydrocolloids: Second Edition, pp. 1–22. (2009)
Rascón-Díaz, M.P., Tejero, J.M.: Spray drying yogurt incorporating hydrocolloids: structural analysis, acetaldehyde content, viable bacteria, and rheological properties. Food Bioprocess Technology, vol. 5, pp. 560–567, 2012. (2012)
Milani, J., Maleki, G.: Hydrocolloids in food industry. Food Industrial Processing vol. In B. Valdez (Ed.), pp. 17–38. (2012)
Mboowa, D.: A review of the traditional pulping methods and the recent improvements in the pulping processes. In: Biomass Conversion and Biorefinery. Springer Science and Business Media Deutschland GmbH (2021)
Espinosa, E., Rol, F., Bras, J., Rodríguez, A.: Production of lignocellulose nanofibers from wheat straw by different fibrillation methods. Comparison of its viability in cardboard recycling process. J. Clean. Prod. 239 (2019). https://doi.org/10.1016/j.jclepro.2019.118083
Desmaisons, J., Boutonnet, E., Rueff, M., Dufresne, A., Bras, J.A.: New Quarafality index for benchmarking of different cellulose nanofibrils. Carbohydr. Polym. 174, 318–329 (2017)
Wibowo, S., Vervoort, L., Tomic, J., Santiago, J.S., Lemmens, L., Panozzo, A., Van Loey, A.: Colour and carotenoid changes of pasteurised orange juice during storage. Food Chem. 171, 330–340 (2015)
Lafarga, T., Álvarez, C., Bobo, G., Aguiló-Aguayo, I.: Characterization of functional properties of proteins from Ganxet beans (Phaseolus vulgaris L. var. Ganxet) isolated using an ultrasound-assisted methodology. LWT. 98, 106–112 (2018)
Coelho, E.M., Gomes, R.G., Machado, B.A.S., Oliveira, R.S., dos Santos Lima, M., de Azêvedo, L.C., Guez, M.A.U.: Passion fruit peel flour–Technological properties and application in food products. Food Hydrocolloids, vol. 62, pp. 158–164, 2017. (2017)
Babbar, N., Aggarwal, P., Oberoi, H.S.: Effect of addition of hydrocolloids on the colloidal stability of litchi (litchi chinensis sonn.) Juice. J. Food Process. Preserv. 39(n– 2), p183–p189 (2015)
Ditzel, F.I., Prestes, E., Carvalho, B.M., Demiate, I.M., Pinheiro, L.A.: Nanocrystalline cellulose extracted from pine wood and corncob. Carbohydr. Polym. 157, 1577–1585 (2017)
Nascimento, D.M., Almeida, J.S., Dias, A.F., Figueirêdo, M.C.B., Morais, J.P.S., Feitosa, J.P., Rosa, M.D.F.: A novel green approach for the preparation of cellulose nanowhiskers from white coir. Carbohydr. Polym. 110, 456–463 (2014)
Vera-Loor, A., Rigou, P., Marlin, N., Mortha, G., Dufresne, A.: Oxidation treatments to convert paper-grade Eucalyptus kraft pulp into microfibrillated cellulose. Carbohydr. Polym. 296, 119946 (2022)
Mnasri, A., Khiari, R., Dhaouadi, H., Halila, S., Mauret, E.: Acidic and alkaline deep eutectic solvents pre-treatment to produce high aspect ratio microfibrillated cellulose. Bioresour. Technol. 368, 128312 (2023)
Nehra, P., Chauhan, R.P.: Facile synthesis of nanocellulose from wheat straw as an agricultural waste. Iran. Polym. J. 31(6), 771–778 (2022)
Costell, E., Clemente, G., Duran, L.: Reología físico química Del puré de albaricoque. II. Caracterización Del flujo y relación entre Los parámetros reológicos y las características químicas y físicas del producto. Revista De Agroquímica Y Tecnología De Aliment. 22(4), 539–550 (1982)
Ibarz, A., Castell-Perez, E., Barbosa-Cánovas, G.V.: Newtonian and Non-Newtonian Flow, p. 11. Food Engineering-Volume II (2009)
Troller, J.: Water activity and food. Academic Press. pp.252. 978-0124335875 (2012)
Bratu, M.G., Popescu, E.C.: Study on the use of thickeners for obtaining low fat mayonnaises. Annals Food Sci. Technol. 17(2), 289–292 (2016)
Amin, M., Elbeltagy, A.E., Mustafa, M., Khalil, A.H.: Development of low fat mayonnaise containing different types and levels of hydrocolloid gum. J. Agroalimental Process. Technol. 20(1), 54–63 (2014)
Sass-Kiss, J., Kiss, P., Milotay, M.M., Kerek, M., Toth-Markus: Differences in anthocyanin and carotenoid content of fruits and vegetables. Food Research International, Volume 38, Issues 8–9, 2005, Pages 1023–1029, ISSN 0963–9969. (2005)
Sinchaipanit, P., Kerr, W.L., Chamchan, R.: Effect of sweeteners and hydrocolloids on quality attributes of reduced-calorie carrot juice. J. Sci. Food. Agric. 93(13), 3304–3311 (2013)
Kandasamy, P., Varadharaju, N., Kalemullah, S., Maladhi, D.: Optimization of process parameters for foam-mat drying of papaya pulp,» Journal of Food Science and Technology, vol. 51, nº 10, pp. 2526–2534. (2012)
Kampf, N., Martinez, C.G., Corradini, M.G., Peleg, M.: Effect of two gums on the development, rheological properties and stability of egg albumen foams. Rheologica Acta, vol. 42, nº 3, pp. 259–268. (2003)
Acknowledgements
The authors would like to thank the AGRIMAX and AGROMATTER project for funding the research. AGRIMAX is funded by the Bio Based Industries Joint Undertaking under the European Union’s Horizon 2020 Research and Innovation Programme under grant agreement No. 720719. Cervera Network AGROMATTER is a strategic research and development program funded by CDTI State Program for the Generation of Knowledge and Scientific and Technological Strengthening of the R&I System, of the State Plan for Scientific and Technical Research and Innovation 2021–2023. This work was also supported by the CERCA Programme of ‘Generalitat de Catalunya’. The authors thank the support of Mariona Segura in the experimental part. I. Aguiló-Aguayo and T. Lafarga thanks the Spanish Ministry of Economy, Industry, and Competitiveness and the European Social Fund for the Postdoctoral Senior Grants Ramon y Cajal (RYC-2016-19949 and RYC2021-031061-I, respectively).
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Consent for Publication
All authors read and approved the final manuscript.
Competing Interests
The authors have declared no conflicts of interest for this research article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aguiló-Aguayo, I., Albaladejo, P., Gallur, M. et al. High Added Value Products from Agroindustrial Residues: Study on Microfibrillated Cellulose for Food Applications. Waste Biomass Valor (2024). https://doi.org/10.1007/s12649-024-02605-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12649-024-02605-1