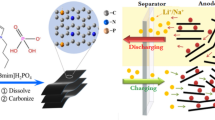
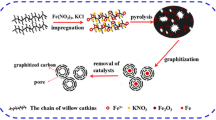
Abstract
Graphitic carbons derived from coconut waste have emerged as interesting candidates for sustainable lithium-ion battery (LIB) anodes. As the demand for high-capacity LIBs, there is a pressing need for graphitic carbon structures that can deliver good performance. However, obtaining good graphitic carbon performance from coconut waste-based material through efficient carbon conversion technology remains a challenge. Hence, we demonstrate that Ni–KOH plays a significant role in the single-pot graphitization process, which effectively generates porous graphitic carbon (PGC) structures. This Ni–KOH single-pot technique reduces the initial formation temperature of graphitic nanostructure from 1200 °C to 800 °C and simultaneously increases the graphitization degree of the carbon product. The resulting sample at 1000 °C (1000-ANi-KOH) exhibits a remarkable reversible capacity of 451.83 mAh/g at 0.05 C when used as LIB anodes. The synergistic effect of a high-order graphitic structure (1.66 IG/ID ratio) and a high BET surface area (599.414 m2/g) contributes to this excellent performance. By providing additional active sites for Li+ adsorption and storage, the porous structure supports high-capacity performance. Finally, these findings point to a realistic strategy for converting Indonesian coconut coir waste into a sustainable carbon source for energy storage materials.
Graphical Abstract








Similar content being viewed by others
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Darjazi, H., Bottoni, L., Moazami, H.R., Rezvani, S.J., Balducci, L., Sbrascini, L., Staffolani, A., Tombesi, A., Nobili, F.: From waste to resources: transforming olive leaves to hard carbon as sustainable and versatile electrode material for Li/Na-ion batteries and supercapacitors. Mater. Today Sustain. 21, 100313 (2023). https://doi.org/10.1016/j.mtsust.2022.100313
Haluska, O., Meščeriakovė, S.M., Murashko, K., Meščeriakovas, A., Kalidas, N., Rantanen, J., Liu, L., Salami, A., Lappalainen, R., Lähde, A., Lehto, V.P., Riikonen, J.: Production of graphitic carbons from plant-based SiC/C nanocomposites for Li-ion batteries. Mater. Chem. Phys. (2023). https://doi.org/10.1016/j.matchemphys.2022.127286
Niu, H., Wang, L., Guan, P., Zhang, N., Yan, C., Ding, M., Guo, X., Huang, T., Hu, X.: Recent advances in application of ionic liquids in electrolyte of lithium ion batteries. J. Energy Storage. 40, 102659 (2021). https://doi.org/10.1016/j.est.2021.102659
Tian, Y., Lai, R., Li, X., Tian, J.: State-of-charge estimation for lithium-ion batteries based on attentional sequence-to-sequence architecture. J. Energy Storage. 62, 106836 (2023). https://doi.org/10.1016/j.est.2023.106836
Costa, C.M., Barbosa, J.C., Gonçalves, R., Castro, H., Campo, F.J.D., Lanceros-Méndez, S.: Recycling and environmental issues of lithium-ion batteries: advances, challenges and opportunities. Energy Storage Mater. 37, 433–465 (2021). https://doi.org/10.1016/j.ensm.2021.02.032
Ali, H., Khan, H.A., Pecht, M.G.: Circular economy of Li batteries: technologies and trends. J. Energy Storage 40, 102690 (2021). https://doi.org/10.1016/j.est.2021.102690
Shi, M., Song, C., Tai, Z., Zou, K., Duan, Y., Dai, X., Sun, J.: Coal-derived synthetic graphite with high specific capacity and excellent cyclic stability as anode material for lithium-ion batteries. Fuel 292, 120250 (2021). https://doi.org/10.1016/j.fuel.2021.120250
Han, L., Zhu, X., Yang, F., Liu, Q., Jia, X.: Eco-conversion of coal into a nonporous graphite for high-performance anodes of lithium-ion batteries. Powder Technol. 382, 40–47 (2021). https://doi.org/10.1016/j.powtec.2020.12.052
R. Pell, P. Whattoff, J. Lindsay, Climate Impact of Graphite Production, Minviro. (2021).
Beyssac, O., Rumble, D.: Graphitic carbon: A ubiquitous, diverse, and useful geomaterial. Elements 10, 415–420 (2014). https://doi.org/10.2113/gselements.10.6.415
Directorate General of Estates, Statistical of National Leading Estate Crops Commodity 2020–2022, Secr. Dir. Gen. Estates. (2022) 1–572.
Sesuk, T., Tammawat, P., Jivaganont, P., Somton, K., Limthongkul, P., Kobsiriphat, W.: Activated carbon derived from coconut coir pith as high performance supercapacitor electrode material. J. Energy Storage. 25, 100910 (2019). https://doi.org/10.1016/j.est.2019.100910
Din, N.A.S., Lim, S.J., Maskat, M.Y., Zaini, N.A.M.: Bioconversion of coconut husk fibre through biorefinery process of alkaline pretreatment and enzymatic hydrolysis. Biomass Convers. Biorefinery. 11, 815–826 (2021). https://doi.org/10.1007/s13399-020-00895-8
Taherian, R., Matboo Ghorbani, M., Kiahosseini, S.R.: A new method for optimal fabrication of carbon composite paper as gas diffusion layer used in proton exchange membrane of fuel cells. J. Electroanal. Chem. 815, 90–97 (2018). https://doi.org/10.1016/j.jelechem.2018.03.009
Destyorini, F., Yudianti, R., Irmawati, Y., Hardiansyah, A., Hsu, Y.I., Uyama, H.: Temperature driven structural transition in the nickel-based catalytic graphitization of coconut coir. Diam. Relat. Mater. 117, 108443 (2021). https://doi.org/10.1016/j.diamond.2021.108443
Deng, J., Xiong, T., Wang, H., Zheng, A., Wang, Y.: Effects of cellulose, hemicellulose, and lignin on the structure and morphology of porous carbons. ACS Sustain. Chem. Eng. 4, 3750–3756 (2016). https://doi.org/10.1021/acssuschemeng.6b00388
Li, S., Song, W.L., Han, X., Cui, Q., Li Zhu, Y., Jiao, S.: Low-temperature graphitization of lignin via Co-assisted electrolysis in molten salt. Green Energ. Environ. (2023). https://doi.org/10.1016/j.gee.2023.04.006
E.I. Akpan, 2019 Sustainable Lignin for Carbon Fibers: Principles, Techniques, and Applications. https://doi.org/10.1007/978-3-030-18792-7.
Zhang, H., Yang, Y., Ren, D., Wang, L., He, X.: Graphite as anode materials: fundamental mechanism, recent progress and advances. Energy Storage Mater. 36, 147–170 (2021). https://doi.org/10.1016/j.ensm.2020.12.027
Destyorini, F., Amalia, W.C., Irmawati, Y., Hardiansyah, A., Priyono, S., Aulia, F., Oktaviano, H.S., Hsu, Y.I., Yudianti, R., Uyama, H.: High graphitic carbon derived from coconut coir waste by promoting potassium hydroxide in the catalytic graphitization process for lithium-ion battery anodes. Energy Fuels 36, 5444–5455 (2022). https://doi.org/10.1021/acs.energyfuels.2c00632
Yu, K., Zhang, Z., Liang, J., Liang, C.: Natural biomass-derived porous carbons from buckwheat hulls used as anode for lithium-ion batteries. Diam. Relat. Mater. 119(108553), 1–11 (2021). https://doi.org/10.1016/j.diamond.2021.108553
Boonprachai, R., Autthawong, T., Namsar, O., Yodbunork, C., Yodying, W., Sarakonsri, T.: Natural porous carbon derived from popped rice as anode materials for lithium-ion batteries. Crystals 12, 1–15 (2022). https://doi.org/10.3390/cryst12020223
Barnakov, C.N., Khokhlova, G.P., Popova, A.N., Sozinov, S.A., Ismagilov, Z.R.: XRD characterization of the structure of graphites and carbon materials obtained by the low-temperature graphitization of coal tar pitch. Eurasian Chem. J. 17, 87–93 (2015). https://doi.org/10.18321/ectj198
Smith, M.W., Dallmeyer, I., Johnson, T.J., Brauer, C.S., McEwen, J.S., Espinal, J.F., Garcia-Perez, M.: Structural analysis of char by Raman spectroscopy: improving band assignments through computational calculations from first principles. Carbon N. Y. 100, 678–692 (2016). https://doi.org/10.1016/j.carbon.2016.01.031
Schuepfer, D.B., Badaczewski, F., Guerra-Castro, J.M., Hofmann, D.M., Heiliger, C., Smarsly, B., Klar, P.J.: Assessing the structural properties of graphitic and non-graphitic carbons by Raman spectroscopy. Carbon N. Y. 161, 359–372 (2020). https://doi.org/10.1016/j.carbon.2019.12.094
Robertson, J.: Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 61, 632–645 (2000). https://doi.org/10.1007/BF02543692
Dennison, J.R., Holtz, M.: Raman spectroscopy of carbon materials. Spectroscopy 11, 38–46 (1996)
Hu, C., Sedghi, S., Silvestre-albero, A., Andersson, G.G., Sharma, A., Pendleton, P., Rodrı, F., Biggs, M.J.: Raman spectroscopy study of the transformation of the carbonaceous skeleton of a polymer-based nanoporous carbon along the thermal annealing pathway. Carbon N. Y. 85, 147–158 (2015). https://doi.org/10.1016/j.carbon.2014.12.098
Eom, Y., Son, S.M., Kim, Y.E., Lee, J.E., Hwang, S.H., Chae, H.G.: Structure evolution mechanism of highly ordered graphite during carbonization of cellulose nanocrystals. Carbon N. Y. 150, 142–152 (2019). https://doi.org/10.1016/j.carbon.2019.05.007
Zhang, S., Liu, Q., Zhang, H., Ma, R., Li, K., Wu, Y., Teppen, B.J.: Structural order evaluation and structural evolution of coal derived natural graphite during graphitization. Carbon N. Y. 157, 714–723 (2020). https://doi.org/10.1016/j.carbon.2019.10.104
Sevilla, M., Fuertes, A.B.: Graphitic carbon nanostructures from cellulose. Chem. Phys. Lett. 490, 63–68 (2010). https://doi.org/10.1016/j.cplett.2010.03.011
Fujimoto, A., Yamada, Y., Koinuma, M., Sato, S.: Origins of sp3C peaks in C1s X-ray photoelectron spectra of carbon materials. Anal. Chem. 88, 6110–6114 (2016). https://doi.org/10.1021/acs.analchem.6b01327
Zhang, X., Zhang, K., Li, H., Wang, Q., Jin, L., Cao, Q.: Synthesis of porous graphitic carbon from biomass by one-step method And its role in the electrode for supercapacitor. J. Appl. Electrochem. 48, 415–426 (2018). https://doi.org/10.1007/s10800-018-1170-x
Li, S., Harris, S., Anandhi, A., Chen, G.: Predicting biochar properties and functions based on feedstock and pyrolysis temperature: a review and data syntheses. J. Clean. Prod. 215, 890–902 (2019). https://doi.org/10.1016/j.jclepro.2019.01.106
Smith, M., Scudiero, L., Espinal, J., McEwen, J.S., Garcia-Perez, M.: Improving the deconvolution and interpretation of XPS spectra from chars by ab initio calculations. Carbon N. Y. 110, 155–171 (2016). https://doi.org/10.1016/j.carbon.2016.09.012
Liu, X., Tao, H., Tang, C., Yang, X.: Anthracite-derived carbon as superior anode for lithium/potassium-ion batteries. Chem. Eng. Sci. 248, 117200 (2022). https://doi.org/10.1016/j.ces.2021.117200
Lua, A.C., Yang, T.: Effect of activation temperature on the textural and chemical properties of potassium hydroxide activated carbon prepared from pistachio-nut shell. J. Colloid Interface Sci. 274, 594–601 (2004). https://doi.org/10.1016/j.jcis.2003.10.001
Gong, Y., Li, D., Luo, C., Fu, Q., Pan, C.: Highly porous graphitic biomass carbon as advanced electrode materials for supercapacitors. Green Chem. 19, 4132–4140 (2017). https://doi.org/10.1039/c7gc01681f
McNamara, K.W., Ayyappan, P., Rajagopalan, R., Chen, J.G., Foley, H.C.: Localized crystallization of polyfurfuryl alcohol derived carbon by alkali metals. Carbon N. Y. 56, 109–120 (2013). https://doi.org/10.1016/j.carbon.2012.12.077
Liu, K., Zhang, J., Ding, R., Zheng, X., Yang, T., Wang, C., Chen, M.: Potassium-assisted carbonization of pyrrole to prepare nanorod-structured graphitic carbon with a high surface area for high-rate supercapacitors. Carbon N. Y. 155, 326–333 (2019). https://doi.org/10.1016/j.carbon.2019.09.005
Raj, K.G., Joy, P.A.: Role of localized graphitization on the electrical and magnetic properties of activated carbon. J. Am. Ceram. Soc. 100, 5151–5161 (2017). https://doi.org/10.1111/jace.15035
C. Chen, K. Sun, A. Wang, S. Wang, J. Jiang, Catalytic graphitization of cellulose using nickel as catalyst, BioResources. 13 (2018) 3165–3176. https://doi.org/10.15376/biores.13.2.3165-3176.
Yan, Q., Li, J., Zhang, X., Hassan, E.B., Wang, C., Zhang, J., Cai, Z.: Catalytic graphitization of kraft lignin to graphene-based structures with four different transitional metals. J. Nanoparticle Res. 20, 1–20 (2018). https://doi.org/10.1007/s11051-018-4317-0
Gomez-Martin, A., Martinez-Fernandez, J., Ruttert, M., Heckmann, A., Winter, M., Placke, T., Ramirez-Rico, J.: Iron-catalyzed graphitic carbon materials from biomass resources as anodes for lithium-ion batteries. Chemsuschem 11, 2776–2787 (2018). https://doi.org/10.1002/cssc.201800831
Li, S.S., Wang, J.K., Zhu, Q., Zhao, X.W., Zhang, H.J.: Fabrication of graphitic carbon spheres via a hydrothermal carbonization combined catalytic graphitization method using cobalt as catalysts. Solid State Phenom. 281, 807–812 (2018). https://doi.org/10.4028/www.scientific.net/SSP.281.807
Gomez-Martin, A., Gutierrez-Pardo, A., Martinez-Fernandez, J., Ramirez-Rico, J.: Binder-free supercapacitor electrodes: optimization of monolithic graphitized carbons by reflux acid treatment. Fuel Process. Technol. 199, 106279 (2020). https://doi.org/10.1016/j.fuproc.2019.106279
Illa, M.P., Sharma, C.S., Khandelwal, M.: Catalytic graphitization of bacterial cellulose–derived carbon nanofibers for stable and enhanced anodic performance of lithium-ion batteries. Mater. Today Chem. 20, 100439 (2021). https://doi.org/10.1016/j.mtchem.2021.100439
Singh, G., Lee, J., Bahadur, R., Karakoti, A., Yi, J., Vinu, A.: Highly graphitized porous biocarbon nanosheets with tunable Micro-Meso interfaces and enhanced layer spacing for CO2 capture and LIBs. Chem. Eng. J. 433, 134464 (2022). https://doi.org/10.1016/j.cej.2021.134464
Wang, K., Cao, Y., Wang, X., Kharel, P.R., Gibbons, W., Luo, B., Gu, Z., Fan, Q., Metzger, L.: Nickel catalytic graphitized porous carbon as electrode material for high performance supercapacitors. Energy 101, 9–15 (2016). https://doi.org/10.1016/j.energy.2016.01.059
Li, J., Zhang, Z., Wang, Z., Cao, Q., Guo, F., Cao, Q.: Low temperature graphitization and electrochemical properties of porous carbon catalyzed with bimetal Ni-Mo. Diam. Relat. Mater. 123, 108862 (2022). https://doi.org/10.1016/j.diamond.2022.108862
Liao, H., Zhong, L., Zeng, H., Xiao, Y., Cheng, B., Lei, S.: A dual-acetate synchronous catalysis-activation strategy towards regulable porous graphitic carbon for high-energy supercapacitor with acetate water-in-salt electrolyte. Carbon N. Y. 213, 118305 (2023). https://doi.org/10.1016/j.carbon.2023.118305
Mishra, S.K., Kanungo, S.B.: Thermal dehydration and decomposition of nickel chloride hydrate (NiCl2·xH2O). J. Therm. Anal. 38, 2417–2436 (1992). https://doi.org/10.1007/BF01974621
Rastegar, H., Mansorizadeh, E.: In situ formed nano-Ni catalytic effect on graphitization of phenolic resin (thermodynamic and microstructure investigation). Carbon Lett. 32, 835–848 (2022). https://doi.org/10.1007/s42823-022-00318-w
Anton, R.: On the reaction kinetics of Ni with amorphous carbon. Carbon N. Y. 46, 656–662 (2008). https://doi.org/10.1016/j.carbon.2008.01.021
Khoshk Rish, S., Tahmasebi, A., Wang, R., Dou, J., Yu, J.: Formation mechanism of nano graphitic structures during microwave catalytic graphitization of activated carbon. Diam. Relat. Mater. 120, 108699 (2021). https://doi.org/10.1016/j.diamond.2021.108699
Placke, T., Siozios, V., Schmitz, R., Lux, S.F., Bieker, P., Colle, C., Meyer, H.W., Passerini, S., Winter, M.: Influence of graphite surface modifications on the ratio of basal plane to “non-basal plane” surface area and on the anode performance in lithium ion batteries. J. Power. Sources 200, 83–91 (2012). https://doi.org/10.1016/j.jpowsour.2011.10.085
Etacheri, V., Wang, C., O’Connell, M.J., Chan, C.K., Pol, V.G.: Porous carbon sphere anodes for enhanced lithium-ion storage. J. Mater. Chem. A. 3, 9861–9868 (2015). https://doi.org/10.1039/c5ta01360g
J.M. Wrogemann, O. Fromm, F. Deckwirth, K. Beltrop, A. Heckmann, M. Winter, T. Placke, 2022 Impact of Degree of Graphitization, Surface Properties and Particle Size Distribution on Electrochemical Performance of Carbon Anodes for Potassium-Ion Batteries, Batter. Supercaps. https://doi.org/10.1002/batt.202200045
Shellikeri, A., Watson, V., Adams, D., Kalu, E.E., Read, J.A., Jow, T.R., Zheng, J.S., Zheng, J.P.: Investigation of pre-lithiation in graphite and hard-carbon anodes using different lithium source structures. J. Electrochem. Soc. 164, A3914–A3924 (2017). https://doi.org/10.1149/2.1511714jes
Xie, L., Tang, C., Bi, Z., Song, M., Fan, Y., Yan, C., Li, X., Su, F., Zhang, Q., Chen, C.: Hard carbon anodes for next-generation li-ion batteries: review and perspective. Adv. Energy Mater. 11, 1–22 (2021). https://doi.org/10.1002/aenm.202101650
Zhang, L., Wang, W., Lu, S., Xiang, Y.: Carbon anode materials: a detailed comparison between Na-ion and K-ion batteries. Adv. Energy Mater. 11, 1–15 (2021). https://doi.org/10.1002/aenm.202003640
Kim, T., Jo, C., Lim, W.G., Lee, J., Lee, J., Lee, K.H.: Facile conversion of activated carbon to battery anode material using microwave graphitization. Carbon N. Y. 104, 106–111 (2016). https://doi.org/10.1016/j.carbon.2016.03.021
Oktaviano, H.S., Waki, K.: Understanding the Li storage sites in MWCNTs: SEI, the Key for Delithiation at high potential. J. Electrochem. Soc. 163, A442–A446 (2016). https://doi.org/10.1149/2.0351603jes
Y.S. Choudhary, L. Jothi, G. 2017 Nageswaran, Electrochemical Characterization, Elsevier Inc. https://doi.org/10.1016/B978-0-323-46140-5.00002-9.
Wang, K., Xu, Y., Wu, H., Yuan, R., Zong, M., Li, Y., Dravid, V., Ai, W., Wu, J.: A hybrid lithium storage mechanism of hard carbon enhances its performance as anodes for lithium-ion batteries. Carbon N. Y. 178, 443–450 (2021). https://doi.org/10.1016/j.carbon.2020.11.095
Qiu, D., Kang, C., Li, M., Wei, J., Hou, Z., Wang, F., Yang, R.: Biomass-derived mesopore-dominant hierarchical porous carbon enabling ultra-efficient lithium ion storage. Carbon N. Y. 162, 595–603 (2020). https://doi.org/10.1016/j.carbon.2020.02.083
Weiss, M., Ruess, R., Kasnatscheew, J., Levartovsky, Y., Levy, N.R., Minnmann, P., Stolz, L., Waldmann, T., Wohlfahrt-Mehrens, M., Aurbach, D., Winter, M., Ein-Eli, Y., Janek, J.: Fast charging of lithium-ion batteries: a review of materials aspects. Adv. Energy Mater. 11(2101126), 1–37 (2021). https://doi.org/10.1002/aenm.202101126
Rao, X., Lou, Y., Chen, J., Lu, H., Cheng, B., Wang, W., Fang, H., Li, H., Zhong, S.: Polyacrylonitrile hard carbon as anode of high rate capability for lithium ion batteries. Front. Energy Res. 8, 1–9 (2020). https://doi.org/10.3389/fenrg.2020.00003
Park, T.H., Yeo, J.S., Seo, M.H., Miyawaki, J., Mochida, I., Yoon, S.H.: Enhancing the rate performance of graphite anodes through addition of natural graphite/carbon nanofibers in lithium-ion batteries. Electrochim. Acta 93, 236–240 (2013). https://doi.org/10.1016/j.electacta.2012.12.124
Acknowledgements
The authors express gratitude to the Japan Society for the Promotion of Science through the JSPS RONPAKU (Dissertation Ph.D.) Program and Research Project from Research Organization for Electronics & Informatics, National Research and Innovation Agency (BRIN)-Indonesia for financial support of this work. The authors acknowledge the facilities and technical support from Advanced Characterization Laboratories in Serpong, Research Center for Advanced Materials at the National Research and Innovation Agency in Indonesia, and Osaka University.
Funding
The authors acknowledge the financial support of the JSPS RONPAKU (Dissertation Ph.D) Program and Research Project from Research Organization for Electronic & Informatics, National Research and Innovation Agency (BRIN) - Indonesia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Destyorini, F., Priyono, S., Oktaviano, H.S. et al. Porous Graphitic Carbon from Coconut Coir Biochar Developed by Ni–KOH Single-Pot Graphitization Process for Lithium-Ion Battery Anodes. Waste Biomass Valor 15, 2881–2895 (2024). https://doi.org/10.1007/s12649-023-02343-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-023-02343-w