Abstract
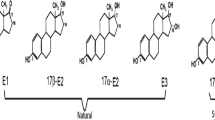
A large amount of estrogen is introduced into the feed to improve livestock growth, excessive residual estrogen would exist in the excrements. The accumulation of estrogen can cause the water and soil pollution around the farm, further inhibit growth and induce caner for organisms and human. Anaerobic digestion (AD) could effectively remove estrogen in livestock manure while the mechanism is still unclear. In this study, the mechanism of estrogen removal by AD was explored during AD process of pig manure. Estrone (E1), estradiol (E2) estriol (E3) and ethinylestradiol (EE2) were selected as research subjects. The removal rates of E1, E2, E3 and EE2 were 19.14, 28.62, 25.83 and 11.81%. Dissolved organic matters (DOM), especially humic acid, play an important role in reducing bioavailability of estrogen. Estrogen will be absorbed by DOM through structures such as aromatic ring and amides. Estrogen may also promote the growth of microorganisms which could degrade estrogen, such as Rhodococcus, Sphingomonas and Pseudomonas. The amount of these microorganisms and dissolved microbial metabolites did not change obviously. This study would give a new explanation for the removal of estrogen and can provide theoretical support for harmless treatment of pig manure.
Graphical Abstract







Similar content being viewed by others
Data Availability
The datasets generated and analyzed during the current study are not publicly available due to time limitations but are available from the corresponding author on reasonable request.
References
Bartelt-Hunt, S.L., Snow, D.D., Kranz, W.L., Mader, T.L., Shapiro, C.A., van Donk, S.J., Shelton, D.P., Tarkalson, D.D., Zhang, T.C.: Effect of growth promotants on the occurrence of endogenous and synthetic steroid hormones on feedlot soils and in runoff from beef cattle feeding operations. Environ. Sci. Technol. 46, 1352–1360 (2012). https://doi.org/10.1021/es202680q
Wei, Z., Wang, J.J., Fultz, L.M., White, P., Jeong, C.: Application of biochar in estrogen hormone-contaminated and manure-affected soils: impact on soil respiration, microbial community and enzyme activity. Chemosphere 270, 128625 (2021). https://doi.org/10.1016/j.chemosphere.2020.128625
Li, C., Li, Y., Li, X., Ma, X., Ru, S., Qiu, T., Lu, A.: Veterinary antibiotics and estrogen hormones in manures from concentrated animal feedlots and their potential ecological risks. Environ. Res. 198, 110463 (2021). https://doi.org/10.1016/j.envres.2020.110463
Conley, J.M., Evans, N., Cardon, M.C., Rosenblum, L., Iwanowicz, L.R., Hartig, P.C., Schenck, K.M., Bradley, P.M., Wilson, V.S.: Occurrence and in vitro bioactivity of estrogen, androgen, and glucocorticoid compounds in a nationwide screen of United States stream waters. Environ. Sci. Technol. 51, 4781–4791 (2017). https://doi.org/10.1021/acs.est.6b06515
Palme, R., Fischer, P., Schildorfer, H., Ismail, M.N.: Excretion of infused 14C-steroid hormones via faeces and urine in domestic livestock. Anim. Reprod. Sci. 43, 43–63 (1996). https://doi.org/10.1016/0378-4320(95)01458-6
Lee, B., Kullman, S.W., Yost, E.E., Worley-Davis, L., Reckhow, K.H.: An object-oriented Bayesian network approach for establishing swine manure-borne natural estrogenic compounds budget. Sci. Total Environ. 639, 815–825 (2018). https://doi.org/10.1016/j.scitotenv.2018.05.209
Douglas, P., Robertson, S., Gay, R., Hansell, A.L., Gant, T.W.: A systematic review of the public health risks of bioaerosols from intensive farming. Int. J. Hyg. Environ. Health 221, 134–173 (2018). https://doi.org/10.1016/j.ijheh.2017.10.019
Tao, H., Zhang, J., Shi, J., Guo, W., Liu, X., Zhang, M., Ge, H., Li, X.: Occurrence and emission of phthalates, bisphenol A, and oestrogenic compounds in concentrated animal feeding operations in Southern China. Ecotoxicol. Environ. Saf. 207, 111521 (2021). https://doi.org/10.1016/j.ecoenv.2020.111521
Zhao, X., Grimes, K.L., Colosi, L.M., Lung, W.-S.: Attenuation, transport, and management of estrogens: a review. Chemosphere 230, 462–478 (2019). https://doi.org/10.1016/j.chemosphere.2019.05.086
Bertin, A., Damiens, G., Castillo, D., Figueroa, R., Minier, C., Gouin, N.: Developmental instability is associated with estrogenic endocrine disruption in the Chilean native fish species, Trichomycterus areolatus. Sci. Total Environ. 714, 136638 (2020). https://doi.org/10.1016/j.scitotenv.2020.136638
Czarny, K., Szczukocki, D., Krawczyk, B., Zieliński, M., Miękoś, E., Gadzała-Kopciuch, R.: The impact of estrogens on aquatic organisms and methods for their determination. Crit. Rev. Environ. Sci. Technol. 47, 909–963 (2017). https://doi.org/10.1080/10643389.2017.1334458
Li, M., Sun, L., Wang, D.: Roles of estrogens in fish sexual plasticity and sex differentiation. Gen. Comp. Endocrinol. 277, 9–16 (2019). https://doi.org/10.1016/j.ygcen.2018.11.015
Liu, S., Gao, H., Dong, Q., Su, Y., Dai, T., Qin, Z., Yang, Y., Gao, Q.: Bacteria are better predictive biomarkers of environmental estrogen transmission than fungi. Environ. Pollut. 298, 118838 (2022). https://doi.org/10.1016/j.envpol.2022.118838
Wocławek-Potocka, I., Mannelli, C., Boruszewska, D., Kowalczyk-Zieba, I., Waśniewski, T., Skarżyński, D.J.: Diverse effects of phytoestrogens on the reproductive performance: cow as a model. Int. J. Endocrinol. 2013, 650984 (2013). https://doi.org/10.1155/2013/650984
Odinga, E.S., Zhou, X., Mbao, E.O., Ali, Q., Waigi, M.G., Shiraku, M.L., Ling, W.: Distribution, ecological fate, and risks of steroid estrogens in environmental matrices. Chemosphere 308, 136370 (2022). https://doi.org/10.1016/j.chemosphere.2022.136370
Khanal, S.K., Xie, B., Thompson, M.L., Sung, S., Ong, S.-K., van Leeuwen, J.: (Hans): fate, transport, and biodegradation of natural estrogens in the environment and engineered systems. Environ. Sci. Technol. 40, 6537–6546 (2006). https://doi.org/10.1021/es0607739
Yang, S., Yu, W., Yang, L., Du, B., Chen, S., Sun, W., Jiang, H., Xie, M., Tang, J.: Occurrence and fate of steroid estrogens in a Chinese typical concentrated dairy farm and slurry irrigated soil. J. Agric. Food Chem. 69, 67–77 (2021). https://doi.org/10.1021/acs.jafc.0c05068
Furuichi, T., Kannan, K., Suzuki, K., Tanaka, S., Giesy, J.P., Masunaga, S.: Occurrence of estrogenic compounds in and removal by a swine farm waste treatment plant. Environ. Sci. Technol. 40, 7896–7902 (2006). https://doi.org/10.1021/es0609598
Zhang, Q., Zhao, J.-L., Ying, G.-G., Liu, Y.-S., Pan, C.-G.: Emission estimation and multimedia fate modeling of seven steroids at the river basin scale in China. Environ. Sci. Technol. 48, 7982–7992 (2014). https://doi.org/10.1021/es501226h
Khan, B., Lee, L.S.: Estrogens and synthetic androgens in manure slurry from trenbolone acetate/estradiol implanted cattle and in waste-receiving lagoons used for irrigation. Chemosphere 89, 1443–1449 (2012). https://doi.org/10.1016/j.chemosphere.2012.06.015
Arcanjo, G.S., dos Santos, C.R., Cavalcante, B.F., de Moura, G.A., Ricci, B.C., Mounteer, A.H., Santos, L.V.S., Queiroz, L.M., Amaral, M.C.S.: Improving biological removal of pharmaceutical active compounds and estrogenic activity in a mesophilic anaerobic osmotic membrane bioreactor treating municipal sewage. Chemosphere 301, 134716 (2022). https://doi.org/10.1016/j.chemosphere.2022.134716
Jarošová, B., Filip, J., Hilscherová, K., Tuček, J., Šimek, Z., Giesy, J.P., Zbořil, R., Bláha, L.: Can zero-valent iron nanoparticles remove waterborne estrogens? J. Environ. Manag. 150, 387–392 (2015). https://doi.org/10.1016/j.jenvman.2014.12.007
Bayode, A.A., dos Santos, D.M., Omorogie, M.O., Olukanni, O.D., Moodley, R., Bodede, O., Agunbiade, F.O., Taubert, A., de Camargo, A.S.S., Eckert, H., Vieira, E.M., Unuabonah, E.I.: Carbon-mediated visible-light clay-Fe2O3–graphene oxide catalytic nanocomposites for the removal of steroid estrogens from water. J. Water Process Eng. 40, 101865 (2021). https://doi.org/10.1016/j.jwpe.2020.101865
Hu, J., Li, T., Zhao, Y., Zhang, X., Ren, H., Huang, H.: A novel in-situ enhancement strategy of denitrification biofilter for simultaneous removal of steroid estrogens and total nitrogen from low C/N wastewater. Chem. Eng. J. 452, 138896 (2023). https://doi.org/10.1016/j.cej.2022.138896
Nghiem, L.D., Manis, A., Soldenhoff, K., Schäfer, A.I.: Estrogenic hormone removal from wastewater using NF/RO membranes. J. Membr. Sci. 242, 37–45 (2004). https://doi.org/10.1016/j.memsci.2003.12.034
Dscenzo, G., Di Corcia, A., Gentili, A., Mancini, R., Mastropasqua, R., Nazzari, M., Samperi, R.: Fate of natural estrogen conjugates in municipal sewage transport and treatment facilities. Sci. Total Environ. 302, 199–209 (2003). https://doi.org/10.1016/S0048-9697(02)00342-X
Paterakis, N., Chiu, T.Y., Koh, Y.K.K., Lester, J.N., McAdam, E.J., Scrimshaw, M.D., Soares, A., Cartmell, E.: The effectiveness of anaerobic digestion in removing estrogens and nonylphenol ethoxylates. J. Hazard. Mater. 199–200, 88–95 (2012). https://doi.org/10.1016/j.jhazmat.2011.10.075
Noguera-Oviedo, K., Aga, D.S.: Chemical and biological assessment of endocrine disrupting chemicals in a full scale dairy manure anaerobic digester with thermal pretreatment. Sci. Total Environ. 550, 827–834 (2016). https://doi.org/10.1016/j.scitotenv.2016.01.084
Neale, P.A., Escher, B.I., Leusch, F.D.L.: Understanding the implications of dissolved organic carbon when assessing antagonism in vitro: an example with an estrogen receptor assay. Chemosphere 135, 341–346 (2015). https://doi.org/10.1016/j.chemosphere.2015.04.084
Liu, R., Wilding, A., Hibberd, A., Zhou, J.L.: Partition of endocrine-disrupting chemicals between colloids and dissolved phase as determined by cross-flow ultrafiltration. Environ. Sci. Technol. 39, 2753–2761 (2005). https://doi.org/10.1021/es0484404
Ping, C.Y., Aiken, G., O’Loughlin, E.: Molecular weight, polydispersity, and spectroscopic properties of aquatic humic substances. Environ. Sci. Technol. 28, 1853–1858 (1994). https://doi.org/10.1021/es00060a015
Jin, X., Hu, J., Ong, S.L.: Influence of dissolved organic matter on estrone removal by NF membranes and the role of their structures. Water Res. 41, 3077–3088 (2007). https://doi.org/10.1016/j.watres.2007.04.025
Yamamoto, H., Liljestrand, H.M., Shimizu, Y., Morita, M.: Effects of physical-chemical characteristics on the sorption of selected endocrine disruptors by dissolved organic matter surrogates. Environ. Sci. Technol. 37, 2646–2657 (2003). https://doi.org/10.1021/es026405w
Goh, J.Y., Goh, K.S., Yip, Y.M., Ng, C.K.: High salinity enhances the adsorption of 17α-ethinyl estradiol by polyethersulfone membrane: isotherm modelling and molecular simulation. Int. J. Environ. Sci. Technol. 19, 5195–5204 (2022). https://doi.org/10.1007/s13762-021-03468-y
Rong, R., Li, Z., Zheng, Y., Zhang, F.: Effect of biochar on 17β-estradiol degradation in composted poultry manure: residue and bioassay analysis. Waste Biomass Valor. 11, 4711–4720 (2020). https://doi.org/10.1007/s12649-019-00788-6
Ye, X., Peng, T., Feng, J., Yang, Q., Pratush, A., Xiong, G., Huang, T., Hu, Z.: A novel dehydrogenase 17β-HSDx from Rhodococcus sp. P14 with potential application in bioremediation of steroids contaminated environment. J. Hazard. Mater. 362, 170–177 (2019). https://doi.org/10.1016/j.jhazmat.2018.09.023
Yu, C.-P., Deeb, R.A., Chu, K.-H.: Microbial degradation of steroidal estrogens. Chemosphere 91, 1225–1235 (2013). https://doi.org/10.1016/j.chemosphere.2013.01.112
Guo, J., Qiu, S., Dai, L., Zhang, L., Meng, L., Liu, M., Yao, H.: The occurrence and removal of steroid estrogens in a full-scale anaerobic/anoxic/aerobic-membrane bioreactor process and the implication of the bacterial community dynamics. J. Environ. Chem. Eng. 10, 107294 (2022). https://doi.org/10.1016/j.jece.2022.107294
Zhang, Y., Snow, D.D., Bartelt-Hunt, S.L.: Stereoselective degradation of estradiol and trenbolone isomers in alluvial sediment. Environ. Sci. Technol. 50, 13256–13264 (2016). https://doi.org/10.1021/acs.est.6b02171
Kumar, V.V., Avisar, D., Prasanna, V.L., Betzalel, Y., Mamane, H.: Rapid visible-light degradation of EE2 and its estrogenicity in hospital wastewater by crystalline promoted g-C3N4. J. Hazard. Mater. 398, 122880–77 (2020). https://doi.org/10.1016/j.jhazmat.2020.122880
Canet-Martí, A., Grüner, S., Lavrnić, S., Toscano, A., Streck, T., Langergraber, G.: Comparison of simple models for total nitrogen removal from agricultural runoff in FWS wetlands. Water Sci. Technol. 85, 3301–3314 (2022). https://doi.org/10.2166/wst.2022.179
Cui, H., Ou, Y., Wang, L., Yan, B., Li, Y., Bao, M.: Critical passivation mechanisms on heavy metals during aerobic composting with different grain-size zeolite. J. Hazard. Mater. 406, 124313 (2021). https://doi.org/10.1016/j.jhazmat.2020.124313
Ma, L., Yates, S.R.: Dissolved organic matter and estrogen interactions regulate estrogen removal in the aqueous environment: a review. Sci. Total Environ. 640–641, 529–542 (2018). https://doi.org/10.1016/j.scitotenv.2018.05.301
Pretorius, L., Smith, C.: Tyramine-induced gastrointestinal dysregulation is attenuated via estradiol associated mechanisms in a zebrafish larval model. Toxicol. Appl. Pharmacol. 461, 116399 (2023). https://doi.org/10.1016/j.taap.2023.116399
Yudt, M.R., Vorojeikina, D., Zhong, L., Skafar, D.F., Sasson, S., Gasiewicz, T.A., Notides, A.C.: Function of estrogen receptor tyrosine 537 in hormone binding, DNA binding, and transactivation. Biochemistry 38, 14146–14156 (1999). https://doi.org/10.1021/bi9911132
Hiroi, R., McDevitt, R.A., Morcos, P.A., Clark, M.S., Neumaier, J.F.: Overexpression or knockdown of rat tryptophan hyroxylase-2 has opposing effects on anxiety behavior in an estrogen-dependent manner. Neuroscience 176, 120–131 (2011). https://doi.org/10.1016/j.neuroscience.2010.12.019
Ren, D., Huang, B., Xiong, D., He, H., Meng, X., Pan, X.: Photodegradation of 17α-ethynylestradiol in dissolved humic substances solution: kinetics, mechanism and estrogenicity variation. J. Environ. Sci. 54, 196–205 (2017). https://doi.org/10.1016/j.jes.2016.03.002
Bedard, M., Giffear, K.A., Ponton, L., Sienerth, K.D., Del Gaizo Moore, V.: Characterization of binding between 17β-estradiol and estriol with humic acid via NMR and biochemical analysis. Biophys. Chem. 189, 1–7 (2014). https://doi.org/10.1016/j.bpc.2014.02.001
Liu, J., Liu, J., Xu, D., Ling, W., Li, S., Chen, M.: Isolation, immobilization, and degradation performance of the 17β-estradiol-degrading bacterium Rhodococcus sp. JX-2. Water Air Soil Pollut. 227, 422 (2016). https://doi.org/10.1007/s11270-016-3122-6
Peng, W., Fu, Y., Jia, B., Sun, X., Wang, Y., Deng, Z., Lin, S., Liang, R.: Metabolism analysis of 17α-ethynylestradiol by Pseudomonas citronellolis SJTE-3 and identification of the functional genes. J. Hazard. Mater. 423, 127045 (2022). https://doi.org/10.1016/j.jhazmat.2021.127045
Budeli, P., Ekwanzala, M.D., Unuofin, J.O., Momba, M.N.B.: Endocrine disruptive estrogens in wastewater: revisiting bacterial degradation and zymoremediation. Environ. Technol. Innov. 21, 101248 (2021). https://doi.org/10.1016/j.eti.2020.101248
Sato, Y., Murakami, T., Funatsuki, H., Matsuba, S., Saruyama, H., Tanida, M.: Heat shock-mediated APX gene expression and protection against chilling injury in rice seedlings. J. Exp. Bot. 52, 145–151 (2001). https://doi.org/10.1093/jexbot/52.354.145
Zheng, J., Hu, M., Zhu, L.: Removal behaviors of aerobic granular sludge on estrogens: adsorption kinetics and removal mechanism. J. Water Process Eng. 44, 102410 (2021). https://doi.org/10.1016/j.jwpe.2021.102410
Tizaoui, C., Fredj, S.B., Monser, L.: Polyamide-6 for the removal and recovery of the estrogenic endocrine disruptors estrone, 17β-estradiol, 17α-ethinylestradiol and the oxidation product 2-hydroxyestradiol in water. Chem. Eng. J. 328, 98–105 (2017). https://doi.org/10.1016/j.cej.2017.07.045
Scott, J.S., Bailey, A., Buttar, D., Carbajo, R.J., Curwen, J., Davey, P.R.J., Davies, R.D.M., Degorce, S.L., Donald, C., Gangl, E., Greenwood, R., Groombridge, S.D., Johnson, T., Lamont, S., Lawson, M., Lister, A., Morrow, C.J., Moss, T.A., Pink, J.H., Polanski, R.: Tricyclic indazoles—a novel class of selective estrogen receptor degrader antagonists. J. Med. Chem. 62, 1593–1608 (2019). https://doi.org/10.1021/acs.jmedchem.8b01837
Sun, R., Song, J., Liu, S.J., Zhao, H., Yan, C.L., Zhang, A.J., Koirala, D., Li, D.W., Hu, C.: Design, synthesis and biological evaluation of 1,4-dihydrothieno[3′,2′:5,6]thiopyrano[4,3-c]pyrazole-3-carboxylic amide derivatives as potential estrogen receptor antagonists. Chin. Chem. Lett. 22, 256–259 (2011). https://doi.org/10.1016/j.cclet.2010.10.029
Acknowledgements
This work was supported by School of Environmental and Engineering, Changzhou University. The authors would like to thank School of Environmental and Engineering, Changzhou University for their effort in laboratory works.
Funding
This research was supported by “Research on key technologies for co-production of organic fertilizers from bio methanol based on biogas and green hydrogen” and “Postgraduate Research and Practice Innovation Program of Jiangsu Province” (grant number BE2022426 and SJCX22_1383).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by BZ, JY. The first draft of the manuscript was written by XL and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luo, X., Zhao, B., Yao, J. et al. Degradation Performance of Estrogen During Anaerobic Digestion of Pig Manure. Waste Biomass Valor 15, 2625–2635 (2024). https://doi.org/10.1007/s12649-023-02286-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-023-02286-2