Abstract
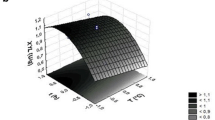
Agribusiness is the main branch of the Brazilian economy. Every year, millions of tons of agro-industrial waste are generated and improperly disposed on soil and water, generating social and environmental impacts. However, these wastes can be used as substrate for fungal growth and to produce enzymes of biotechnological interest (hydrolases, proteases, lipases). In this study, we present the production of an endoglucanase from Pycnoporus sanguineus (the first enzyme isolated from this organism) by solid-state fermentation of agro-industrial wastes. Endoglucanase was produced after 72 fermentation hours, and was isolated via ethanol fractionation and ion exchange chromatography. The isolated enzyme had an optimum temperature of 50 °C, thermostability between 30 and 60 °C, optimum pH of 5.0, stability at pH values from 5.0 to 8.0, KM of 3.18 ± 0.005 mg/mL, and kcat of 4.53 s−1. It also displayed halophilic properties. Additionally, endoglucanase was able to saccharify three kinds of agro-industrial waste in 24 h without any chemical or physical pretreatment. Rice husk, wheat bran, and sugarcane bagasse produced 273, 192, and 155 mg/mL of reducing sugars after enzymatic hydrolysis, respectively. Thus, enzyme shows to be a promising option for obtaining fermentable sugars from agro-industrial waste for the production of second-generation ethanol.
Graphical Abstract








Similar content being viewed by others
Data Availability
Enquiries about data availability should be directed to the authors.
References
Diaz, A.B., Blandino, A., Caro, I.: Value added products from fermentation of sugars derived from agro-food residues. Thends Food Sci. Techonol. 71, 52–64 (2018). https://doi.org/10.1016/j.tifs.2017.10.016
Marzo, C., Díaz, A.B., Caro, I., Blandino, A.: Valorization of agro-industrial wastes to produce hydrolytic enzymes by fungal solid-state fermentation. Waste Manage Res. 37(2), 149–156 (2019). https://doi.org/10.1177/0734242X18798
Lopes, L.S., Vieira, A.N., Da Luz, J.M.R., Da Silva, M.C.S., Cardoso, W.S., Kasuya, M.C.M.: Production of fungal enzymes in Macaúba coconut and enzymatic degradation of textile dye. Biocatal. Agric. Biotechnol. 26, 101651–101657 (2020). https://doi.org/10.1016/j.bcab.2020.101651
Silva, T.P., Albuquerque, F.S., Santos, C.W.V., Franco, M., Caetano, L.C., Pereira, H.J.V.: Production, purification, characterization and application of a new halorolerant and thermostable endoglucanase of Botrytis ricni URM 5627. Bioresour. Technol. 270, 263–269 (2018). https://doi.org/10.1016/j.biortech.2018.09.022
Salomão, G.S.B., Agnezi, J.C., Paulino, L.B., Hencker, L.B., Lira, T.S., Tardioli, P.W., Pinotti, L.M.: Production of celulases by solid state fermentation using natural and pretreated sugarcane bagasse with diferente fungi. Biocatal. Agric. Biotechnol. 17, 1–6 (2019). https://doi.org/10.1016/j.bcab.2018.10.019
Santos, P.S., Solidade, L.S., Souza, J.G.B., Lima, G.S., Braga, A.C.R., Assis, F.G.V., Leal, P.L.: Solid-state fermentation in agroindustrial residues for enzymes production: a systematic review. J. Eng. Exact. Sci. 4, 0181–0188 (2018). https://doi.org/10.18540/jcecvl4iss2pp0181-0188
Fasim, A., More, V.S., More, S.S.: Large-scale production of enzymes for biotechnology uses. Curr. Opin. Biotechnol. 69, 68–76 (2021). https://doi.org/10.1016/j.copbio.2020.12.002
Madhavan, A., Arun, K.B., Sindhu, R., Jose, A.A., Pugazhendhi, A., Binod, P., Sirohi, R., Reshmy, R., Awasthi, M.K.: Engineering interverntions in industrial filamentous fungal cell factores for biomass valorization. Bioresour. Technol. 344, 126209 (2022)
Santos, C.W.V., Marques, M.E.C., Tenório, H.A., Miranda, E.C., Pereira, H.J.V.: Purification and characterization of trypsin from Luphiosilurus alexandri pyloric cécum. Biochem. Biophys. Rep. 8, 29–33 (2016). https://doi.org/10.1016/j.bbrep.2016.08.003
Huang, X., Men, P., Tang, S., Lu, X.: Aspergillus terréus as an industrial filamentous fungus for pharmaceutical biotechnology. Curr. Opin. Biotechnol. 69, 273–280 (2021). https://doi.org/10.1016/j.copbio.2021.02.004
Young, D., Dollhofer, V., Callaghan, T.M., Reitberger, S., Michael, L., Benz, J.P.: Isolation, identification and characterization of lignocellulolytic aerobic and anaerobic fungi in one—and two-phase biogas plants. Bioresour. Technol. 268, 470–479 (2018)
Alokika, A., Kumar, A., Kumar, V., Singh, B.: Cellulosic and hemicellulosic fractions of sugarcane bagasse: potential, challenges and future perspective. Int. J. Biol. Macromol. 169, 564–582 (2021). https://doi.org/10.1016/j.ijbiomac.2020.12.175
Das, A., Paul, T., Halder, S.K., Jana, A., Maity, C., Mohapatra, P.K., Pati, B.R., Mondal, K.C.: Production of cellulolytic enzymes by Aspergillus fumigatus ABK9 in wheat bran-rice straw mixed substrate and use of cocktail enzymes for deinking of waste office paper pulp. Bioresour. Technol. 128, 290–296 (2013)
Lin, W., Jia, G., Sun, H., Sun, T., Hou, D.: Genome sequence of the fungus Pycnoporus sanguineus, which produces cinnabarinic acid and pH and thermos-stable laccases. Gene 742, 144586 (2020)
Niderhaus, C., Garrido, M., Insani, M., Campos, E., Wirth, S.: Heterologous production and characterization of a thermostable GH10 family endo-xylanase from Pycnoporus sanguineus BAFC 2126. Process Biochem. 67, 92–98 (2018). https://doi.org/10.1016/j.procbio.2018.01.017
Lee, H.M., Chao, H.C., Lu, M.K.: Effect of carbohydrate-based media on the biomass, polysaccharides molecular weight distribution and sugar composition from Pycnoporus sanguineus. Biomass. Bioenerg. 47, 37–43 (2012)
Bautista, E.G., Gutierrez, E., Dupuy, N., Gaime-Perraud, I., Ziarelli, F., da Silva, A.M.F.: Pre-treatment of a sugarcane bagasse-based substrate prior to saccharification: effect of coffee pulp and urea on laccase and cellulase activities of Pycnoporus sanguineus. J Environ Manage 239, 178–186 (2019). https://doi.org/10.1016/j.jenvman.2019.03.033
Pinar, O., Koçhan, B., Sayar, N.A., Karaosmanoğlu, K., Kazan, D.: Enzyme mixture production from Pycnoporus sanguineus DMSZ 3024 using a lignocellulosic waste, Hazelnut husk: a case study for laccase and cellulose. New Biotechnol. 31, S92 (2014). https://doi.org/10.1016/j.nbt.2014.05.1827
Vikineswary, S., Abdullah, N., Renuvathani, M., Sekaran, M., Pandey, A., Jones, E.B.G.: Productivity of laccase in solid substrate fermentation of selected agro-residues by Pycnoporus sanguineus. Bioresour. Technol. 97, 171–177 (2006). https://doi.org/10.1016/j.biortech.2005.02.015
Orlandelli, R.C., Alberto, R.N., Rubin, C.J., Pamphile, J.A.: Diversity of endophytic fungal community associated with Piper hispidum Sw. (Piperaceae) leaves. Genet. Mol. Res. 11, 1575–1585 (2012). https://doi.org/10.4238/2012
Li, Q., Yi, L., Marek, P., Iverson, L.B.: Commercial proteases: present and future. FEBS Lett. 587, 1155–1163 (2013)
Wang, H.C., Chen, Y.C., Hseu, R.S.: Purification and characterization of a cellulolytic multienzyme complex produced by Neocallimastix patriciarum J11. Biochem. Biophys. Res Commun. 451, 190–195 (2014)
Santos, F.A., Gonçalves, L.C.T.C., Simões, A.L.C.C., Santos, S.F.M.: Evaluation of the production of celluloses by Penicilluim sp FSDE15 using corncob and wheat bran as substrates. Bioresour. Technol. Rep. 14, 100648 (2021)
Silva, T.P., Albuquerque, F.S., Ferreira, A.N., Santos, D.M.R.C., Santos, T.V., Meneghetti, S.M.P., Franco, M., Luz, J.M.R., Pereira, H.J.V.: Dilute-acid pretreatment for enhancing the enzymatic saccharification of agro-residues using a endoglucanase. Biotechnol. Appl. Biochem. 69, 15 (2022). https://doi.org/10.1002/bab.2341
Silva, T.P., Ferreira, A.N., Albuquerque, F.S., Barros, A.C.A., da Luz, J.M.R., Gomes, F.S., Pereira, H.J.V.: Box-Behnken experimental design for the optimization of enzymatic saccharification of wheat bran. Biomass Convers Biorefin 11, 5597–5604 (2021). https://doi.org/10.1007/s13399-021-01378-0
Miller, G.L.: Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal. Chem. 31, 426–428 (1959). https://doi.org/10.1021/ac60147a030
Ghose, T.K.: Measurement of cellulase activities. Pure Appl. Chem. 59, 257–268 (1987). https://doi.org/10.1351/pac198759020257
Bradford, M.: A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 72, 248–254 (1976). https://doi.org/10.1016/0003-2697(76)90527-3
Laemmli, U.K.: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 (1970). https://doi.org/10.1038/227680a0
Ázar, R.I.S.L., Morgan, T., Barbosa, M.H.P., Guimarães, V.M., Ximenes, E., Ladisch, M.: Impact of protein blocking on enzymatic saccharification of bagasse from sugarcane clones. Biotechnol. Bioeng. 116, 1584–1593 (2018). https://doi.org/10.1002/bit.26962
Bi, Z., Zhao, Y., Morrell, J.J., Lei, Y., Yan, L.: The antifungal mechanism of konjac flying poder extract and its active compounds against wood decay fungi. Ind. Crops. Prod. 164, 113406 (2021)
Prado, J.M., Forster-Carneiro, T., Rostagno, M.A., Follegatti-romero, L.A., Filho, F.M., Meireles, M.A.A.: Obtaining sugars from coconut husk, defatted grape seed, and pressed palm fiber by hydrolysis with subcritical water. J. Supercrit. Fluids 89, 89–98 (2014). https://doi.org/10.1016/j.supflu.2014.02.017
Houfani, A.A., Anders, N., Spiess, A.C., Baldrian, P., Benallaoua, S.: Insights from enzymatic degradation of celulose and hemicelulose to fermentable sugarsv: a review. Biomass. Bioenergy 134, 105481 (2020)
Solomons, T.W.G., Craig, B.F., Scott, A.S.: Organic chemistry. Wiley India; 12º ed, 2019
Gomathi, D., Muthulakshmi, C., Kumar, G.D., Ravikumar, G., Kalaiselvi, M., Uma, C.: Submerged fermentation of wheat bran by Aspergillus flavus for production and characterization of carboxy methyl cellulase. Asian Pacific J Trop. Biomed. S67–S73 (2012)
El-Khonezy, M.I., Elgammal, E.W., Ahmed, E.F., Elaziz, A.M.A.: Detergent stable thiol-dependant alcaline protease produced from the endophytic fungus Aspergillus ochraceus BT21: Purification and kinetics. Biocatal. Agric. Biotechnol. 35, 102046 (2021)
Xue, D.S., Zheng, X., Lin, D., Yao, S.: Ethanol tolerant endoglucanase from Aspergillus niger isolated from wine fermentation cellar. Biocatal. Agric. Biotechnol. 15, 19–24 (2018). https://doi.org/10.1016/j.bcab.2018.04.016
Jia, Y., Xue, Z., Wang, Y., Lu, Y., Li, R., Li, N., Wang, Q., Zhang, M., Chen, H.: Chemical structure and inhbition on α-glucosidase of polysaccharides from corn silk by fractional precipitation. Carbohydr. Polym. 252,(2021). https://doi.org/10.1016/j.carbpol.2020.117185
Bohorquez, M. A. M. Ethanol precipitation of glycosyl hydrolases produced by Trichoderma harzianum P49P11 [in Portuguese]. Universidade Estadual de Campinas, 71 p., (2014).
Han, C., Liu, Y., Liu, M., Wang, S., Wang, Q.: Improving the thermostability of a thermostable endoglucanase from Chaetomium thermophilum by engineering the conserved noncatalytic residue and N-glycosylation site. Int. J. Biol. Macromol. 164, 3361–3368 (2020)
Narra, M., Dixit, G., Divecha, J., Kumar, K., Madamwar, D.: Production, purification and characterization of a novel GH 12 family endoglucanase from Aspergillus terreus and its application in enzymatic degradation of delignified rice straw. Int. Biodeterior. Biodegrad. 88, 150–161 (2014)
Asha, P., Jose, D., Singh, I.S.B.: Purification and characterization of processive-type endoglucanase and β-glucosidase from Aspergillus ochraceus MTCC 1810 through saccharification of delignified coir pith to glucose. Bioresour. Technol 213, 245–248 (2016)
Dixit, M., Shukla, P.: Multi-efficient endoglucanase from Aspergillus niger MPS25 and its potential applications in saccharification of wheat straw and waste paper deinking. Chemosphere 313, 137298 (2023). https://doi.org/10.1016/j.chemosphere.2022.137298
Massarente, V.S., Zanoni, J.A., Gomes, E., Rodriguez, G.O.B.: Biochemical characterization of endoglucanases produced by Myceliophthora thermophila M77 in solid-state culture. Biocatal. Agric. Biotechnol. 27, 101684 (2020). https://doi.org/10.1016/j.bcab.2020.101684
Liu, Z., Li, J., Jie, C., Wu, B., Hao, N.: A multifunctional α-amylase BSGH13 from Bacillus subtilis BS-5 possessing endoglucanase and xylanase activities. Int. J. Biol. Macromol. 171, 166–176 (2021). https://doi.org/10.1016/j.ijbiomac.2021.01.003
Litthauer, D., Vuuren, M.J.V., Tonder, A.V., Wolfaardt, F.W.: Purification and kinetics of a thermostable laccase from Pycnoporus sanguineus (SCC 108). Enzyme Microb. Technol. 40(4), 563–568 (2007). https://doi.org/10.1016/j.enzmictec.2006.05.011
Wang, Z., Liu, J., Ning, Y., Liao, X., Jia, Y.: Eichhornia crassipes: Agro-waster for a novel thermostable laccase production by Pycnoporus sanguineus SYBC-L1. J. Biosci. Bioeng. 123, 163–169 (2017). https://doi.org/10.1016/j.jbiosc.2016.09.005
Juturu, V., Wu, J.C.: Microbial cellulases: Engineering, production and applications. Renew. Sust. Energy Rev. 33, 188−203 (2014). https://doi.org/10.1016/j.rser.2014.01.077
Dadwal, A., Sharma, S., Satyanarayana, T.: Thermostable celulose saccharifying microbial enzymes: characteristics, recente advances and biotechnological applications. Int. J. Biol. Macromol. 188, 226–244 (2022)
Dotsenko, A.S., Rozhkova, A.M., Zorov, I.N., Sinitsyn, A.P.: Protein surfarce engineering of endoglucanase Penicillium verruculosum for improvement in thermostability and stability in the presence of 1-butyl-3-methylimidazolium chloride ionic liquid. Bioresour. Technol. 296, 122370 (2020)
Almeida, M.N., Falkoski, D.L., Guimarães, V.M., Ramos, H.J., Visser, E.M., Alfenas, M.G.P., Rezende, S.T.: Characteristic of free endoglucanase and glycosidases multuenzyme complex from Fusarium verticillioides. Bioresour. Technol. 143, 413–422 (2013)
Dhar, H., Kasana, R.C., Gulati, A.: Heterologous expression and characterization of detergente stable endoglucanase EG5B from Paenibacillus sp. IHB 3084. J. Mol. Catal. B 120, 9–15 (2015). https://doi.org/10.1016/j.molcatb.2015.06.009
Madern, D., Ebel, C., Zaccai, G.: Halophilic adaptation of enzymes. Extremophiles 4, 91–98 (2000)
García, M.D., Urdiales, B.V., González, C.N.A., Esquivel, J.C.C., Herrera, R.R.: Halophilic hydrolases as a new tool for the biotechnological industries. J. Sci. Food Agric. 92, 2575–2580 (2012)
Mokashe, N., Chaudhari, B., Patil, U.: Operative utility of salt-stable proteases of halophilic and halotolerante bacteria in the biotechnology sector. Int. J. Biol. Macromol. 117, 493–522 (2018)
Ghio, S., Bradanini, M.B., Garrido, M.M., Ontanon, O.M., Piccinni, F.E., Villegas, R.M.D., Talia, P.M., Campos, E.: Synergic activity of Cel8Pa β-1–4 endoglucanase and Bg1Pa β-glucosidase from Paenibacillus xylanivorans A59 in beta-glucan conversion. Biotechnol. Rep. 28, 00526 (2020). https://doi.org/10.1016/j.btre.2020.e00526
Karp, S.G., Rozhkova, A.M., Semenova, M.V., Osipov, D.O., Pauli, S.T.Z., Sinitsyna, O.A., Zorov, I.N., Vandenberghe, L.P.S., Soccol, C.R., Sinitsyn, A.P.: Designing enzyme cocktails from Penicillium and Aspergillus species for the enhaced saccharification of agro-industrial wastes. Bioresour. Technol. 330, 124888 (2021). https://doi.org/10.1016/j.biortech.2021.124888
Safak, H., Otur, Ç., Kizildogan, A.K.: Molecular and biochemical characterization of a recombinant endoglucanase rCKT3eng, from an extreme halophilic Haloarcula sp. strain CKT3. Int. J. Biol. Macromol. 151, 1173–1180 (2020). https://doi.org/10.1016/j.ijbiomac.2019.10.161
Patel, A., Shah, A.: Purification and characterization of novel, thermostable and non-processive GH5 family endoglucanase from Fomitopsis meliae CFA 2. Int. J. Biol. Macromol. 182, 1161–1169 (2021). https://doi.org/10.1016/j.ijbiomac.2021.04.110
Abraham, A., Mathew, A.K., Sindhu, R., Pandey, A., Binod, P.: Potential of rice straw for bio-refining: an overview. Bioresour. Technol. 215, 29–36 (2016). https://doi.org/10.1016/j.biortech.2016.04.011
Tanaskovic, J.S., Sekuljica, N., Jovanovic, J., Gazikalovic, I., Grbavcic, S., Dordevic, N., Sekulic, M.V., Hao, J., Lukovic, N., Jugovic, K.Z.: Upgrading of valuable food componente contentes and anti-nutritional factors depletion by solid-state fermentation: a way to valorize wheat bran for nutrition. J. Cereal. Sci. 99, 103159 (2021)
Bertonha, L.C., Neto, M.L., Garcia, J.A.A., Vieira, T.F., Castoldi, R., Bracht, A., Peralta, R.M.: Screening of Fusarium sp. for xylan and cellulose hydrolyzing enzymes and perspectives for the saccharification of delignified sugarcane bagasse. Biocatal. Agric. Biotechnol. 16, 385–389 (2018)
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors have not disclosed any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
dos Santos, D.M.R.C., Albuquerque, F., Silva, T.P. et al. Production, Purification, Characterization, and Application of Halotolerant and Thermostable Endoglucanase Isolated from Pycnoporus sanguineus. Waste Biomass Valor 14, 3211–3222 (2023). https://doi.org/10.1007/s12649-023-02175-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-023-02175-8