Abstract
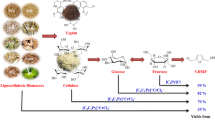
Direct conversion of lignocellulosic biomass into 5-ethoxymethylfurfural (5-EMF); a promising fuel alternative and biofuel additive, is achieved by using acidic ionic liquids (AILs). A number of AILs have been reported to date for biorefinery applications such as dissolution, deconstruction and saccharification of lignocellulosic biomass as well as conversion into platform chemicals such as 5-hydroxymethylfurfural (5-HMF) and levulinic acid. Designing suitable ionic liquid (IL) that is efficient in terms of cost as well as product selectivity is still highly needed. This study is carried out to scrutinize the biomass-to-5-EMF conversion efficiencies of different Brønsted acidic ionic liquids (BAILs) as well as Brønsted Lewis acidic ionic liquids (BLAILs) based on pyridinium (Py), 1,4-diazoniabicyclo[2.2.2]octane (DABCO) and 1,1,3,3-tetramethylguanidinium (TMG) cations. Among all tested ILs, chloroaluminate of [DABCO]HSO4 showed the highest fructose to 5-EMF conversion potential. High 5-EMF yields up to 56, 66 and 45% are obtained from fructose using Py, DABCO and TMG based BLAILs under the optimized conditions. Moreover, the competency of the system is further authenticated by processing glucose and wheat husk biomass; 42% 5-EMF is obtained from wheat husk using chlorochromate tethered [Py]HSO4. Product was characterized via HPLC and 1H NMR spectroscopy.
Graphical Abstract










Similar content being viewed by others
Data Availability
Supporting data is provided in supporting information file.
References
Asim, A.M., Uroos, M., Naz, S., Sultan, M., Griffin, G., Muhammad, N., Khan, A.S.: Acidic ionic liquids: promising and cost-effective solvents for processing of lignocellulosic biomass. J. Mol. Liq. 287, 110943 (2019)
Naz, S., Uroos, M., Muhammad, N.: Effect of molecular structure of cation and anions of ionic liquids and co-solvents on selectivity of 5-hydroxymethylfurfural from sugars, cellulose and real biomass. J. Mol. Liq. 334, 116523 (2021)
Thombal, R.S., Jadhav, V.H.: Application of glucose derived magnetic solid acid for etherification of 5-HMF to 5-EMF, dehydration of sorbitol to isosorbide, and esterification of fatty acids. Tetrahedron Lett. 57, 4398–4400 (2016)
Gruter, G.J.M., Manzer, L.E.: Hydroxymethylfurfural ethers from sugars or HMF and mixed alcohols, in, US 2010/0058650 A1 (2010)
Mascal, M., Nikitin, E.B.: Direct, High-yield conversion of cellulose into biofuel. Angew. Chem. 120, 8042–8044 (2008)
Gruter, G.J.M., Dautzenberg, F.: Method for the synthesis of 5-alkoxymethyl furfural ethers and their use, in, US 2011/0082304 A1 (2012)
Alipour, S., Omidvarborna, H., Kim, D.-S.: A review on synthesis of alkoxymethyl furfural, a biofuel candidate. Renew. Sustain. Energy Rev. 71, 908–926 (2017)
Gruter, G.J.M., Dautzenberg, F.: Method for the synthesis of 5-hydroxymethylfurfural ethers and their use, in, US 20110082304 A1 (2014)
Lew, C.M., Rajabbeigi, N., Tsapatsis, M.: One-pot synthesis of 5-(ethoxymethyl) furfural from glucose using Sn-BEA and Amberlyst catalysts. Ind. Eng. Chem. Res. 51, 5364–5366 (2012)
Balakrishnan, M., Sacia, E.R., Bell, A.T.: Etherification and reductive etherification of 5-(hydroxymethyl) furfural: 5-(alkoxymethyl) furfurals and 2, 5-bis (alkoxymethyl) furans as potential bio-diesel candidates. Green Chem. 14, 1626–1634 (2012)
Yu, X., Gao, X., Peng, L., He, L., Zhang, J.: Intensified 5-ethoxymethylfurfural production from biomass components over aluminum-based mixed-acid catalyst in co-solvent medium. ChemSelect 3, 13391–13399 (2018)
Liu, B., Zhang, Z., Huang, K., Fang, Z.: Efficient conversion of carbohydrates into 5-ethoxymethylfurfural in ethanol catalyzed by AlCl3. Fuel 113, 625–631 (2013)
Yang, Y., Hu, C., Abu-Omar, M.M.: Conversion of glucose into furans in the presence of AlCl3 in an ethanol–water solvent system. Bioresour. Technol. 116, 190–194 (2012)
Zhou, X., Zhang, Z., Liu, B., Zhou, Q., Wang, S., Deng, K.: Catalytic conversion of fructose into furans using FeCl3 as catalyst. J. Ind. Eng. Chem. 20, 644–649 (2014)
Liu, B., Zhang, Z.: One-pot conversion of carbohydrates into 5-ethoxymethylfurfural and ethyl D-glucopyranoside in ethanol catalyzed by a silica supported sulfonic acid catalyst. RSC Adv. 3, 12313–12319 (2013)
Wang, J., Zhang, Z., Jin, S., Shen, X.: Efficient conversion of carbohydrates into 5-hydroxylmethylfurfan and 5-ethoxymethylfurfural over sufonic acid-functionalized mesoporous carbon catalyst. Fuel 192, 102–107 (2017)
Liu, A., Liu, B., Wang, Y., Ren, R., Zhang, Z.: Efficient one-pot synthesis of 5-ethoxymethylfurfural from fructose catalyzed by heteropolyacid supported on K-10 clay. Fuel 117, 68–73 (2014)
Ren, Y., Liu, B., Zhang, Z., Lin, J.: Silver-exchanged heteropolyacid catalyst (Ag1H2PW): an efficient heterogeneous catalyst for the synthesis of 5-ethoxymethylfurfural from 5-hydroxymethylfurfural and fructose. J. Ind. Eng. Chem. 21, 1127–1131 (2015)
Che, P., Lu, F., Zhang, J., Huang, Y., Nie, X., Gao, J., Xu, J.: Catalytic selective etherification of hydroxyl groups in 5-hydroxymethylfurfural over H4SiW12O40/MCM-41 nanospheres for liquid fuel production. Bioresour. Technol. 119, 433–436 (2012)
Zhang, Z., Wang, Y., Fang, Z., Liu, B.: Synthesis of 5-ethoxymethylfurfural from fructose and inulin catalyzed by a magnetically recoverable acid catalyst. ChemPlusChem 79, 233–240 (2014)
Yuan, Z., Zhang, Z., Zheng, J., Lin, J.: Efficient synthesis of promising liquid fuels 5-ethoxymethylfurfural from carbohydrates. Fuel 150, 236–242 (2015)
Liu, A., Zhang, Z., Fang, Z., Liu, B., Huang, K.: Synthesis of 5-ethoxymethylfurfural from 5-hydroxymethylfurfural and fructose in ethanol catalyzed by MCM-41 supported phosphotungstic acid. J. Ind. Eng. Chem. 20, 1977–1984 (2014)
Antunes, M.M., Russo, P.A., Wiper, P.V., Veiga, J.M., Pillinger, M., Mafra, L., Evtuguin, D.V., Pinna, N., Valente, A.A.: Sulfonated graphene oxide as effective catalyst for conversion of 5-(hydroxymethyl)-2-furfural into biofuels. Chemsuschem 7, 804–812 (2014)
Lanzafame, P., Barbera, K., Papanikolaou, G., Perathoner, S., Centi, G., Migliori, M., Catizzone, E., Giordano, G.: Comparison of H+ and NH4+ forms of zeolites as acid catalysts for HMF etherification. Catal. Today 304, 97–102 (2018)
Xiang, Y., Wen, S., Tian, Y., Zhao, K., Guo, D., Cheng, F., Xu, Q., Liu, X., Yin, D.: Efficient synthesis of 5-ethoxymethylfurfural from biomass-derived 5-hydroxymethylfurfural over sulfonated organic polymer catalyst. RSC Adv. 11, 3585–3595 (2021)
Kraus, G.A., Guney, T.: A direct synthesis of 5-alkoxymethylfurfural ethers from fructose via sulfonic acid-functionalized ionic liquids. Green Chem. 14, 1593–1596 (2012)
Zuo, M., Le, K., Feng, Y., Xiong, C., Li, Z., Zeng, X., Tang, X., Sun, Y., Lin, L.: An effective pathway for converting carbohydrates to biofuel 5-ethoxymethylfurfural via 5-hydroxymethylfurfural with deep eutectic solvents (DESs). Ind. Crops Prod. 112, 18–23 (2018)
Guo, H., Duereh, A., Hiraga, Y., Aida, T.M., Qi, X., Smith, R.L., Jr.: Perfect recycle and mechanistic role of hydrogen sulfate ionic liquids as additive in ethanol for efficient conversion of carbohydrates into 5-ethoxymethylfurfural. Chem. Eng. J. 323, 287–294 (2017)
Alam, M.I., De, S., Khan, T.S., Haider, M.A., Saha, B.: Acid functionalized ionic liquid catalyzed transformation of non-food biomass into platform chemical and fuel additive. Ind. Crops Prod. 123, 629–637 (2018)
Bing, L., Zhang, Z., Deng, K.: Efficient one-pot synthesis of 5-(ethoxymethyl) furfural from fructose catalyzed by a novel solid catalyst. Ind. Eng. Chem. Res. 51, 15331–15336 (2012)
Yin, S., Sun, J., Liu, B., Zhang, Z.: Magnetic material grafted cross-linked imidazolium based polyionic liquids: an efficient acid catalyst for the synthesis of promising liquid fuel 5-ethoxymethylfurfural from carbohydrates. J. Mater. Chem. A 3, 4992–4999 (2015)
Naz, S., Uroos, M., Ayoub, M.: Cost-effective processing of carbon-rich materials in ionic liquids: an expeditious approach to biofuels. ACS Omega 6, 29233–29242 (2021)
Dutta, S., De, S., Alam, M.I., Abu-Omar, M.M., Saha, B.: Direct conversion of cellulose and lignocellulosic biomass into chemicals and biofuel with metal chloride catalysts. J. Catal. 288, 8–15 (2012)
Naz, S., Uroos, M.: Ionic Liquids mediated one-pot synthesis of second generation 5-Ethoxymethylfurfural (5-EMF), a potent biofuel candidate. ChemSelect 7, e202201161 (2022)
Asim, A.M., Uroos, M., Naz, S., Muhammad, N.: Pyridinium protic ionic liquids: effective solvents for delignification of wheat straw. J. Mol. Liq. 325, 115013 (2021)
Li, C., Zhang, Z., Zhao, Z.K.: Direct conversion of glucose and cellulose to 5-hydroxymethylfurfural in ionic liquid under microwave irradiation. Tetrahedron Lett. 50, 5403–5405 (2009)
Wang, N., Zhang, J., Wang, H., Li, Q., Wang, D.: Effects of metal ions on the hydrolysis of bamboo biomass in 1-butyl-3-methylimidazolium chloride with dilute acid as catalyst. Bioresour. Technol. 173, 399–405 (2014)
Fitzpatrick, S.W.: The Biofine technology: a” bio-refinery” concept based on thermochemical conversion of cellulosic biomass. ACS Symp. Ser. 921, 271–287 (2006)
Yu, X., Gao, X., Tao, R., Peng, L.: Insights into the metal salt catalyzed 5-ethoxymethylfurfural synthesis from carbohydrates. Catalysts 7, 182 (2017)
Xu, G., Chang, C., Fang, S., Ma, X.: Cellulose reactivity in ethanol at elevate temperature and the kinetics of one-pot preparation of ethyl levulinate from cellulose. Renew. Energy 78, 583–589 (2015)
Chen, B., Xu, G., Chang, C., Zheng, Z., Wang, D., Zhang, S., Li, K., Zou, C.: Efficient one-pot production of biofuel 5-ethoxymethylfurfural from corn stover: optimization and kinetics. Energy Fuels 33, 4310–4321 (2019)
Moreno-Recio, M., Santamaría-González, J., Maireles-Torres, P.: Brönsted and Lewis acid ZSM-5 zeolites for the catalytic dehydration of glucose into 5-hydroxymethylfurfural. Chem. Eng. J. 303, 22–30 (2016)
Karnjanakom, S., Phanthong, P., Bayu, A., Maneechakr, P., Samart, C., Kongparakul, S., Guan, G.: Facile in situ 5-EMF synthesis and extraction processes from catalytic conversion of sugar under sustainable long-life cycle. ACS Sustain. Chem. Eng. 8, 14867–14876 (2020)
Quereshi, S., Ahmad, E., Pant, K.K., Dutta, S.: Insights into microwave-assisted synthesis of 5-ethoxymethylfurfural and ethyl levulinate using tungsten disulfide as a catalyst. ACS Sustain. Chem. Eng. 8, 1721–1729 (2019)
Hu, X., Wu, L., Wang, Y., Song, Y., Mourant, D., Gunawan, R., Gholizadeh, M., Li, C.-Z.: Acid-catalyzed conversion of mono-and poly-sugars into platform chemicals: effects of molecular structure of sugar substrate. Bioresour. Technol. 133, 469–474 (2013)
Yang, Y., Abu-Omar, M.M., Hu, C.: Heteropolyacid catalyzed conversion of fructose, sucrose, and inulin to 5-ethoxymethylfurfural, a liquid biofuel candidate. Appl. Energy 99, 80–84 (2012)
Guo, H., Duereh, A., Hiraga, Y., Qi, X., Smith, R.L., Jr.: Mechanism of glucose conversion into 5-ethoxymethylfurfural in ethanol with hydrogen sulfate ionic liquid additives and a lewis acid catalyst. Energy Fuels 32, 8411–8419 (2018)
Naz, S., Uroos, M., Muhammad, N.: One-pot production of 5-hydroxymethylfurfural and simultaneous lignin recovery from non-food lignocellulosic wastes using cost-effective ionic liquids. Biomass Convers. Bioref. 1–12 (2022)
Funding
This work was financially supported by Higher Education Commission (HEC) Pakistan, under the NRPU Research Grant, project no. 8639/Punjab/NRPU/R&D/HEC/2017 and TDF Research Grant, Project no. TDF03-294.
Author information
Authors and Affiliations
Contributions
SN performed the experimental work, validated the analyses data and wrote the manuscript. MU supervised the work and assured the funding and instrumental analyses for the project.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Naz, S., Uroos, M. Synthesis of 5-Ethoxymethylfurfural (5-EMF) from Sugars and Lignocellulosic Crop Residue Using Ionic Liquids Differing in Their Cationic Structure. Waste Biomass Valor 14, 2225–2236 (2023). https://doi.org/10.1007/s12649-022-02010-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-022-02010-6