Abstract
Java plum pomace is rich in bioactive compounds and has potential to use in numerous ways, where extraction is one of them. Ultrasound assisted extraction (UAE) using acidified aqueous ethanol (1:20 solid:liquid, w/v) acidified with 0.1% HCl was employed to optimize the extraction conditions (ultrasound power, extraction temperature, time, and ethanol concentration) for phytochemicals from java plum fruit pomace using response surface methodology. The mathematical model suggested a high coefficient of determination for optimum conditions as 366.25 W ultrasound power, 37.61 °C temperature for 47.48 min using 70% ethanol for the extraction of phytochemicals. Actual yield of phytochemicals was almost same to predicted yields. While comparing UAE with conventional extraction (CE), phytochemical constituents, antioxidant activities, and minerals except potassium, copper, and manganese were reported higher in UAE as confirmed using HPLC, FTIR, AES and SEM analysis. It can be concluded that the optimized conditions can be used for the better extraction of phytochemicals from java plum pomace and its effective utilization.
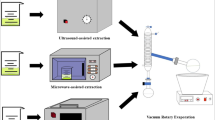
Graphical Abstract




Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are included in this article.
References
Mazzoni, L., Perez-Lopez, P., Giampieri, F., Alvarez-Suarez, J.M., Gasparrini, M., Forbes-Hernandez, T.Y., Quiles, J.L., Mezzetti, B., Battino, M.: The genetic aspects of berries: from field to health. J. Sci. Food Agric. 96, 365–371 (2016). https://doi.org/10.1002/jsfa.7216
Kaur, N., Aggarwal, P., Kumar, V., Kaur, S.: Influence of different extraction techniques on the extraction of phytochemicals and antioxidant activities from Syzygium cumini (jamun) pomace using Taguchi orthogonal array design: a qualitative and quantitative approach. Biomass Convers. Biorefin. (2022). https://doi.org/10.1007/s13399-022-02826-1
Kaur, N., Aggarwal, P.: Development and characterization of packing, microstructural, physico- and phytochemical attributes of potential functional jamun (Syzygium cumini) pomace powder for direct compression: high antioxidant nutraceutical tablets. Int. J. Food Sci. Technol. (2022). https://doi.org/10.1111/ijfs.15933
Pietta, P.G.: Flavonoids as antioxidants. J. Nat. Prod. 63, 1035–1042 (2000). https://doi.org/10.1021/np9904509
Suradkar, N.G., Pawar, V.S., Shere, D.M.: Physicochemical, proximate and bioactive composition of jamun (Syzygium cuminii L.) fruit. Int. J. Chem. Stud. 5, 470–472 (2017). https://doi.org/10.1007/s13197-011
Espada-Bellido, E., Ferreiro-González, M., Carrera, C., Palma, M., Barroso, C.G., Barbero, G.F.: Optimization of the ultrasound-assisted extraction of anthocyanins and total phenolic compounds in mulberry (Morus nigra) pulp. Food Chem. 219, 23–32 (2017). https://doi.org/10.1016/j.foodchem.2016.09.122
Chemat, F., Rombaut, N., Sicaire, A.G., Meullemiestre, A., Fabiano-Tixier, A.S., Abert-Vian, M.: Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 34, 540–560 (2017). https://doi.org/10.1016/j.ultsonch.2016.06.035
Oancea, S., Grosu, C., Ketney, O., Stoia, M.: Conventional and ultrasound-assisted extraction of anthocyanins from blackberry and sweet cherry cultivars. Acta Chim. Slov. 60, 383–389 (2013)
Qin, B., Liu, X., Cui, H., Ma, Y., Wang, Z., Han, J.: Aqueous two-phase assisted by ultrasound for the extraction of anthocyanins from Lycium ruthenicum Murr. Prep. Biochem. Biotechnol. 47, 881–888 (2017). https://doi.org/10.1080/10826068.2017.1350980
Vázquez-Espinosa, M., de Peredo, A.V.G., Ferreiro-González, M., Carrera, C., Palma, M., Barbero, G.F., Espada-Bellido, E.: Assessment of ultrasound assisted extraction as an alternative method for the extraction of anthocyanins and total phenolic compounds from maqui berries (Aristotelia chilensis (Mol.) Stuntz). Agronomy 9, 1–17 (2019). https://doi.org/10.3390/agronomy9030148
Aliaño-González, M.J., Jarillo, J.A., Carrera, C., Ferreiro-González, M., Álvarez, J.Á., Palma, M., Ayuso, J., Barbero, G.F., Espada-Bellido, E.: Optimization of a novel method based on ultrasound-assisted extraction for the quantification of anthocyanins and total phenolic compounds in blueberry samples (Vaccinium corymbosum L.). Foods 9, 1763 (2020). https://doi.org/10.3390/foods9121763
Dibazar, R., Bonat Celli, G., Brooks, M.S.L., Ghanem, A.: Optimization of ultrasound-assisted extraction of anthocyanins from lowbush blueberries (Vaccinium angustifolium Aiton). J. Berry Res. 5, 173–181 (2015). https://doi.org/10.3233/JBR-150100
Khan, M.K., Abert-Vian, M., Fabiano-Tixier, A.S., Dangles, O., Chemat, F.: Ultrasound-assisted extraction of polyphenols (flavanone glycosides) from orange (Citrus sinensis L.) peel. Food Chem. 119, 851–858 (2010). https://doi.org/10.1016/j.foodchem.2009.08.046
Aadil, R.M., Zeng, X.A., Han, Z., Sun, D.W.: Effects of ultrasound treatments on quality of grapefruit juice. Food Chem. 141, 3201–3206 (2013). https://doi.org/10.1016/j.foodchem.2013.06.008
Carrera, C., Ruiz-Rodríguez, A., Palma, M., Barroso, C.G.: Ultrasound assisted extraction of phenolic compounds from grapes. Anal. Chim. Acta 732, 100–104 (2012). https://doi.org/10.1016/j.aca.2011.11.032
Jabbar, S., Abid, M., Wu, T., Hashim, M.M., Saeeduddin, M., Hu, B., Lei, S., Zeng, X.: Ultrasound-assisted extraction of bioactive compounds and antioxidants from carrot pomace: a response surface approach. J. Food Process. Preserv. 39, 1878–1888 (2015). https://doi.org/10.1111/jfpp.12425
Agnieszka, M., Michał, S., Robert, K.: Selection of conditions of ultrasound-assisted, three-step extraction of ellagitannins from selected berry fruit of the rosaceae family using the response surface methodology. Food Anal. Methods 13, 1650–1665 (2020). https://doi.org/10.1007/s12161-020-01762-y
Hanula, M., Wyrwisz, J., Moczkowska, M., Horbańczuk, O.K., Pogorzelska-Nowicka, E., Wierzbicka, A.: Optimization of microwave and ultrasound extraction methods of açai berries in terms of highest content of phenolic compounds and antioxidant activity. Appl. Sci. 10, 1–12 (2020). https://doi.org/10.3390/app10238325
Bamba, B.S.B., Shi, J., Tranchant, C.C., Xue, S.J., Forney, C.F., Lim, L.T.: Influence of extraction conditions on ultrasound-assisted recovery of bioactive phenolics from blueberry pomace and their antioxidant activity. Molecules 23, 1–17 (2018). https://doi.org/10.3390/molecules23071685
Ryu, D., Koh, E.: Application of response surface methodology to acidified water extraction of black soybeans for improving anthocyanin content, total phenols content and antioxidant activity. Food Chem. 261, 260–266 (2018). https://doi.org/10.1016/j.foodchem.2018.04.061
Ang, Y.K., Sia, W.C., Khoo, H.E., Yim, H.S.: Antioxidant potential of Carica papaya peel and seed. Focus Mod. Food Ind. 1, 11–16 (2012)
Papoutsis, K., Pristijono, P., Golding, J.B., Stathopoulos, C.E., Bowyer, M.C., Scarlett, C.J., Vuong, Q.V.: Screening the effect of four ultrasound-assisted extraction parameters on hesperidin and phenolic acid content of aqueous citrus pomace extracts. Food Biosci. 21, 20–26 (2018). https://doi.org/10.1016/j.fbio.2017.11.001
Kaur, N., Aggarwal, P., Kaur, N., Kaur, S.: Nutritional improvement of Bhujia by incorporating coloured bell peppers. J. Food Process. Preserv. (2022). https://doi.org/10.1111/jfpp.16569
Kaur, S., Aggarwal, P., Kaur, N.: Formulations for preparation of ‘Aloo bhujia’ from medium and high sugar potato varieties. Agric. Res. J. 56, 288 (2019). https://doi.org/10.5958/2395-146x.2019.00045.0
Aggarwal, P., Kaur, S., Kaur, N.: Intermediate moisture kinnow bar from low grade kinnow mandarins: phytonutritional profile, morphological characterization, and storage stability. Food Biosci. (2022). https://doi.org/10.1016/j.fbio.2022.101837
Stuart, B.H.: Infrared Spectroscopy: Fundamentals and Applications. Wiley, Chichester (2004)
Esclapez, M.D., García-Pérez, J.V., Mulet, A., Cárcel, J.A.: Ultrasound-assisted extraction of natural products. Food Eng. Rev. 3, 108–120 (2011). https://doi.org/10.1007/s12393-011-9036-6
Kidak, R., Ince, N.H.: Ultrasonic destruction of phenol and substituted phenols: a review of current research. Ultrason. Sonochem. 13, 195–199 (2006). https://doi.org/10.1016/j.ultsonch.2005.11.004
Chaves, J.O., de Souza, M.C., da Silva, L.C., Lachos-Perez, D., Torres-Mayanga, P.C., da Fonseca Machado, A.P., Forster-Carneiro, T., Vázquez-Espinosa, M., González-de-Peredo, A.V., Barbero, G.F., Rostagno, M.A.: Extraction of flavonoids from natural sources using modern techniques. Front. Chem. 8, 507887 (2020). https://doi.org/10.3389/fchem.2020.507887
Cao, X., Cai, C., Wang, Y., Zheng, X.: Effects of ultrasound processing on physicochemical parameters, antioxidants, and color quality of bayberry juice. J. Food Qual. (2019). https://doi.org/10.1155/2019/7917419
Tan, M.C., Tan, C.P., Ho, C.W.: Effects of extraction solvent system, time and temperature on total phenolic content of henna (Lawsonia inermis) stems. Int. Food Res. J. 20, 3117–3123 (2013)
Wang, W., Jung, J., Tomasino, E., Zhao, Y.: Optimization of solvent and ultrasound-assisted extraction for different anthocyanin rich fruit and their effects on anthocyanin compositions. LWT 72, 229–238 (2016). https://doi.org/10.1016/j.lwt.2016.04.041
Goula, A.M., Thymiatis, K., Kaderides, K.: Valorization of grape pomace: drying behavior and ultrasound extraction of phenolics. Food Bioprod. Process. 100, 132–144 (2016). https://doi.org/10.1016/j.fbp.2016.06.016
Sun, C., Wu, Z., Wang, Z., Zhang, H.: Effect of ethanol/water solvents on phenolic profiles and antioxidant properties of Beijing propolis extracts. Evid. Based Complement. Altern. Med. (2015). https://doi.org/10.1155/2015/595393
González-Centeno, M.R., Comas-Serra, F., Femenia, A., Rosselló, C., Simal, S.: Effect of power ultrasound application on aqueous extraction of phenolic compounds and antioxidant capacity from grape pomace (Vitis vinifera L.): experimental kinetics and modeling. Ultrason. Sonochem. 22, 506–514 (2015). https://doi.org/10.1016/j.ultsonch.2014.05.027
Kaderides, K., Goula, A.M., Adamopoulos, K.G.: A process for turning pomegranate peels into a valuable food ingredient using ultrasound-assisted extraction and encapsulation. Innov. Food Sci. Emerg. Technol. 31, 204–215 (2015). https://doi.org/10.1016/j.ifset.2015.08.006
Fu, X., Belwal, T., Cravotto, G., Luo, Z.: Sono-physical and sono-chemical effects of ultrasound: primary applications in extraction and freezing operations and influence on food components. Ultrason. Sonochem. 60, 104726 (2020). https://doi.org/10.1016/j.ultsonch.2019.104726
De Luna, S.L.R., Ramírez-Garza, R.E., Serna Saldívar, S.O.: Environmentally friendly methods for flavonoid extraction from plant material: impact of their operating conditions on yield and antioxidant properties. Sci. World J. (2020). https://doi.org/10.1155/2020/6792069
Karunanithi, A., Venkatachalam, S.: Ultrasonic-assisted solvent extraction of phenolic compounds from Opuntia ficus-indica peel: phytochemical identification and comparison with soxhlet extraction. J. Food Process Eng. (2019). https://doi.org/10.1111/jfpe.13126
Hu, A.J., Hao, S.T., Zheng, J., Chen, L., Sun, P.P.: Multi-frequency ultrasonic extraction of anthocyanins from blueberry pomace and evaluation of its antioxidant activity. J. AOAC Int. 104, 811–817 (2021). https://doi.org/10.1093/jaoacint/qsaa150
Galván D’Alessandro, L., Dimitrov, K., Vauchel, P., Nikov, I.: Kinetics of ultrasound assisted extraction of anthocyanins from Aronia melanocarpa (black chokeberry) wastes. Chem. Eng. Res. Des. 92, 1818–1826 (2014). https://doi.org/10.1016/j.cherd.2013.11.020
Ivanovic, J., Tadic, V., Dimitrijevic, S., Stamenic, M., Petrovic, S., Zizovic, I.: Antioxidant properties of the anthocyanin-containing ultrasonic extract from blackberry cultivar “Čačanska Bestrna.” Ind. Crops Prod. 53, 274–281 (2014). https://doi.org/10.1016/j.indcrop.2013.12.048
Sun, Y., Qiao, L., Ye, X., Liu, D., Zhang, X., Huang, H.: The sonodegradation of caffeic acid under ultrasound treatment: relation to stability. Molecules 18, 561–573 (2013). https://doi.org/10.3390/molecules18010561
Qiao, L., Ye, X., Sun, Y., Ying, J., Shen, Y., Chen, J.: Sonochemical effects on free phenolic acids under ultrasound treatment in a model system. Ultrason. Sonochem. 20, 1017–1025 (2013). https://doi.org/10.1016/j.ultsonch.2012.12.007
Jabbar, S., Abid, M., Hu, B., Wu, T., Hashim, M.M., Lei, S., Zhu, X., Zeng, X.: Quality of carrot juice as influenced by blanching and sonication treatments. LWT 55, 16–21 (2014). https://doi.org/10.1016/j.lwt.2013.09.007
Pereira, V.A., de Arruda, I.N.Q., Stefani, R.: Active chitosan/PVA films with anthocyanins from Brassica oleraceae (red cabbage) as time-temperature indicators for application in intelligent food packaging. Food Hydrocoll. 43, 180–188 (2015). https://doi.org/10.1016/j.foodhyd.2014.05.014
Zhou, P., Wang, X., Liu, P., Huang, J., Wang, C., Pan, M., Kuang, Z.: Enhanced phenolic compounds extraction from Morus alba L. leaves by deep eutectic solvents combined with ultrasonic-assisted extraction. Ind. Crops Prod. 120, 147–154 (2018). https://doi.org/10.1016/j.indcrop.2018.04.071
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conceptualization: [NK, PA]; Methodology: [NK]; Formal analysis and investigation: [NK]; Writing—original draft preparation: [NK]; Writing—review and editing: [NK, VK, SK]; Funding acquisition: [PA]; Resources: [PA]; Supervision: [PA].
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaur, N., Aggarwal, P., Kumar, V. et al. Ultrasound-Assisted Extraction of Phytochemicals from Java Plum (Syzygium cumini L.) Pomace: Process Optimization, Phytochemical Characterization Using HPLC, FTIR, SEM and Mineral Profiling. Waste Biomass Valor 14, 949–961 (2023). https://doi.org/10.1007/s12649-022-01915-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-022-01915-6