Abstract
Purpose
Chitin purification from remains (pupal exuviae after metamorphosis to adult flies) of Hermetia illucens farming was optimized performing demineralization, deproteinization and bleaching under different conditions. The optimal parameters to obtain high-purity chitin were determined.
Methods
Dried and ground pupal exuviae, whose composition was initially determined, were demineralized using six different acids. Proteins were removed with a NaOH treatment in which temperature, molarity and duration were varied in a randomized experiment. Bleaching was carried out testing ten different chemicals, including NaOCl, H2O2, solvent mixtures and enzymes. The efficiency of each step was determined to assess the optimal conditions for each of them. The resulting chitin was subjected to spectroscopic characterization.
Results
The highest demineralization efficiency (90%) was achieved using 0.5 M formic acid for 2 h at 40 °C, confirming the validity of organic acids as a more sustainable alternative to inorganic acids. The treatment with 1.25 M NaOH at 90 °C for 4 h showed the highest deproteinization efficiency, removing 96% of the proteins. Temperature and NaOH concentration were the significant parameters for deproteinization efficiency. The most efficient bleaching treatment was with 6% NaOCl at 60 °C for 1 h (67% efficiency). H2O2 could also be a valid alternative to avoid environmental risk related to chlorine-containing compounds. At the end of the purification process 17% of the original biomass was retained with a chitin content of 85%, corresponding to a chitin yield of 14% related to the initial biomass. Solid-state nuclear magnetic resonance showed that the purified chitin had a degree of acetylation of 96% and X-ray powder diffraction gave a crystallinity index of 74%.
Conclusion
This investigation shows an optimized method for extraction of high-purity chitin from H. illucens pupal exuviae, supporting the validity of insect-farming remains as source of this versatile biopolymer.
Graphical Abstract

Similar content being viewed by others
Statement of Novelty
The industrial production of insect-based proteins and lipids by the conversion of agro-industrial waste streams gained momentum in the last decade. To create a higher value of this process, a valorization of the side streams thereof is required. To this end, we describe the production of pure chitin from one of the major insect processing waste streams, the pupal exuviae. Chitin, as a parental polysaccharide of the acid-soluble chitosan, is currently the focus of comprehensive research for the substitution of synthetic polymers in various application fields. Since we addressed the decentralized extraction of this versatile natural polysaccharide from an insect processing waste stream, our investigations thus provided the basis for a prospective commercialization of this novel approach.
Introduction
Chitin is the Earth’s second most abundant polysaccharide after cellulose, being the main structural component of arthropod’s exoskeleton and the cell wall of fungi [1]. It is a polymer of increasing commercial potential. It is used in agriculture for the biological control of pathogens [2], in tissue regeneration [3], for wound dressing, for adsorption of dyes and heavy metals from waste water, as moisturizing agent in cosmetics, and as additive in paper production [4]. Furthermore, chitin can be converted into chitosan, a polymer with increased solubility and reactivity provided by free amino groups, which can find a wider range of applications [5, 6].
On an industrial scale, chitin is mostly obtained from waste streams of the fishing industry (mainly crustacean’s exoskeleton), whose chitin content ranges between 15 and 40% [7]. Nevertheless, in recent years it has become necessary to look for alternative sources to cope with the huge increase in demand expected for chitin and chitosan [8]. Moreover, crustacean waste does not have a constant availability throughout the year, its supply is becoming no more sustainable without worsening the depletion of marine resources and the chitin quality is not consistent since the raw materials composition varies [9, 10].
Alternative sources of chitin are fungi and insects. Fungi, as for example the species Mucor rouxii, can incorporate chitin (as well as chitosan) up to 40% of the cell wall’s dry weight [11]. Although offering a non-seasonable source of chitin and chitosan, the content varies greatly with the fungal life cycle [12]. Insect exuviae can contain up to 35% chitin of the dry weight, depending on the species [13]. This is a chitin-rich substrate whose availability is increasing, as insect farming for feed production and waste management is growing worldwide. Bioconversion of organic waste and agricultural by-products by insects is one of the technologies that has received the most attention in recent years [14]. Insect waste deriving from this process can be a cost-free source of chitin available throughout the year with low environmental impact [15].
The black soldier fly (Hermetia illucens L.) is one of the most commonly bred species for the insect-based bioconversion. The larval stages of this fly can consume a wide variety of organic substrates converting them into valuable products, such as proteins and fat for animal feed, lipids for biodiesel and cosmetics production, and frass for soil improvement [16].
The life cycle of H. illucens is divided in five developmental stages, including egg, larvae, prepupae, pupae and adult. During transition from larvae to pupae, several larval exoskeletons are shed. Pupal exuviae are released by metamorphosis from pupae to adult flies [17]. As chitin is one of the major components of these by-products of the life cycle [18], they are suitable for a prospective decentralized chitin isolation. The larval exoskeletons are commonly a component of the insect frass, which is a commercial product. The chitin presence in the frass supports growth of beneficial microbes in soil and acts as a biostimulant [19]. Pupal exuviae are easier to collect from H. illucens farming, since they self-collect in the containers used for the development of pupae into adults, and the one that is most readily available. Hence, a process for chitin purification from pupal exuviae should be developed.
To date, the majority of studies focused on the optimization of chitin extraction from fishery waste, for which several methods are available in literature. Purification of chitin from insects still remains on laboratory scale, mainly applying the protocols reported for crustaceans that involve sequential steps to remove minerals, proteins, pigments and catecholic compounds becoming noticeable by the dark colour of the insect cuticle. A comprehensive review of methods for chitin and chitosan purification from insects is provided by Hahn et al. [20]. Chitin purification from pupal exuviae has already been in the focus of a study. However, the objective of Brigode et al. [21] was to obtain physico-chemical data rather than to optimize chitin purification. Furthermore, in most cases whole insects at different developmental stages of their life cycle (f. e. pupae, adult) are used [22,23,24,25,26,27] for chitin purification and investigation instead of side streams from insect farming.
The present work was performed to optimize systematically the chitin purification from side streams of H. illucens breeding, namely pupal exuviae for the first time. Purification steps, i.e. demineralization, deproteinization and bleaching, were carried out under different conditions in order to determine the optimal parameters. The efficiency of demineralization was investigated in relation to the acid used, molarity and final washing volume. The deproteinization step with alkali was optimized concerning temperature, molarity and incubation time. The final bleaching treatment was evaluated concerning the chemical used, duration and temperature.
Materials and Methods
Material
Pupal exuviae are side streams of the black soldier fly (H. illucens) cultivation, manually collected and provided by Protix (Dongen, Netherlands). Insects were reared on exclusively plant origin by-products from the food processing industry. The raw biomass used was initially crushed with an ultra-centrifugal mill (ZM 100, Retsch, Germany) equipped with a ring sieve (Hole diameter: 0.5 mm). Material was stored at − 18 °C until usage.
If not stated otherwise, desalted water was used. Reagents were of practical grade and purchased either from Carl Roth GmbH (Germany) or Merck KgaA (Germany). The commercial chitin purified from Pandalus borealis was purchased from Chitinor AS (Norway) and applied for analytical comparison purposes.
Analytical Methods
The basic analyses were performed as triplicates. The chitin content of the raw material, the demineralized and deproteinized pupal exuviae and the bleached chitin was measured as acid detergent fiber (ADF). The value of the acid detergent lignin (ADL) was measured and deducted from ADF to consider the catechol content within the biomass according to Hahn et al. [18]. The method includes the removal of non-fibrous contents from the homogenized and degreased raw material using an acidic detergent solution at elevated temperatures. After washing, subsequent separation and drying, the residue was weighed and the mass related to the original biomass applied (ADF). To measure the ADL, the residual biomass is subjected to an incubation with concentrated sulfuric acid. Again the residual solid was washed until pH neutrality, dried and weighed. The mass of the ash after incineration of the biomass was subtracted from this value and the difference is to be related to the biomass applied for the measurement (ADL). The difference between the values (ADF − ADL) refers to the chitin content.
The lipid content of the raw material was estimated by Soxhlet extraction with n-hexane as solvent according to Matissek et al. [28]. The moisture and ash contents were determined at 105 °C and 550 °C according to DIN EN 15935:2009 and 12880:2000. Determination of different metal ion contents in the raw material was performed via Inductively Coupled Plasma Optical Emission Spectrometry (Activa M, Horiba Scientific, Japan) after biomass hydrolysis with sulfuric acid. All spectroscopic characterizations were only carried out with hydrogen peroxide (see experiment IV, Table 2) bleached pupal exuviae chitin.
Protein Quantification
A quantification of the proteins in the supernatant via Bradford assay or similar is aggravated by the presence of the catecholic compounds. Hence, the deproteinization conditions were assessed by the amino acid content in the biomass before and after the alkaline treatment. The amino acids were thus measured in the supernatant after total hydrolysis of the biomass. The decomposition was performed with 120 mg sample suspended in 6 mL 6 M HCl incubated for 45 min in a microwave oven at 110 °C. After incubation, water was added up to a volume of 10 mL. The supernatant was removed and D-Norvalin was added as internal standard. Automated precolumn Fluorenylmethoxycarbonyl (Fmoc) derivatization enables the detection of the amino acids in the pmol/µL-range. Separation of the sample was performed with a 1290 Infinity UHPLC-System and a Zorbax-Eclipse-RRHD-column (Agilent Technologies, US) applying a gradient with variable amount of 10 mM Na2HPO4–Na2B4O7–buffer pH 8.2 (mobile phase A) together with Acetonitrile/MetOH/water (45/45/10 v/v/v, mobile Phase B). Volumetric flow rate was adjusted to 0.8 mL/min and the temperature set up to 40 °C. Derivatized amino acids were quantified with a fluorescence detector (266/305 ex/em).
The protein content of the raw material, the demineralized pupal exuviae and the deproteinized samples was determined by summarizing the contents of all amino acids in the biomass detected in the supernatant after total hydrolysis (see Supplementary Material). To perform this, we have to assume indefinite long protein chains, considering the loss of water during peptide bond formation by subtraction of the molecular weight (18 mol/g) from each amino acid. Furthermore, we hypothesize that some amino acids undergo partial or total degradation during hydrolysis [29] so that the real protein content should be slightly higher.
Attenuated Total Reflectance (ATR) Fourier Transform Infrared Spectroscopy (FT-IR)
The FT-IR spectra were recorded on a “Vertex70” spectrometer (Bruker) using a diamond ATR crystal. The measurements were executed in the range of 4000–400 cm−1 with a resolution of 4 cm−1 and a measuring time of 60 scans (> 25 s).
X-ray Diffraction (XRD)
The chitin was filled into mark-tubes (Ø = 7 mm). The XRD measurements were performed on a Bruker AXS Nanostar with a copper anode (λ = 1.5418 Ǻ) and a Histar 2D detector to obtain the scattering intensity as a function of the scattering angle, the obtained 2D images were integrated over one direction in space. The scattering angle was calibrated using silver behenate. The measurement was performed at 25 °C. The crystallinity index (CI) was determined by:
where I110 is the maximum intensity of the (110) lattice diffraction at 2θ = 19° and Iam is the intensity of the amorphous diffraction at 2θ = 12.6° [30, 31].
Solid-State Nuclear Magnetic Resonance (ss-NMR)
1H–13C cross polarized magic-angle spinning (CP-MAS) NMR experiments were performed on an Avance III 400WB spectrometer at a resonance frequency of 100.6 MHz, a spin rate of 8 kHz and by applying a contact time of 1 ms as in literature [32]. A delay of 5 s between the scans was applied.
The degree of acetylation (DA) was calculated from the integral of the methyl carbon C8 divided by the integrals of the ring carbons C1–C6 [32, 33].
Purification Investigations
First, demineralization investigation was carried out (step 1). Identification of a suitable demineralization agent resulted in the production of a larger batch to carry out the deproteinization experiments (step 2). Bleaching was performed as third and final process step
Step 1: Demineralization
For demineralization (DM), a suitable agent needs to be identified from different organic and inorganic acids. For this purpose, H3PO4, H2SO4, HNO3, HCl, CH3COOH, HCOOH were applied. Thus, 1 g of homogenized pupal exuviae were suspended in 15 mL of the respective acid (all 0.5 M with exception of 0.25 M H2SO4) using 50 mL reaction vessels of a parallel synthesis apparatus (Synthesis 1, Heidolph, Germany). The investigations were performed for 2 h at 500 rpm either at 40 or 70 °C. Water incubation without acid addition was carried out at 70 °C to set-up a benchmark. After incubation, the suspension was centrifuged (10 min, 4696 g) and the supernatant was decanted. Then, the residual biomass was washed with 15 mL water and again centrifuged. Wash water was decanted, biomass dried at 105 °C overnight and the ash content was finally measured. All experiments were carried out in triplicates. Demineralization efficiency (DME) was calculated according to the following equation:
whereby ACDem and ACRM is the ash content after and before demineralization, respectively.
Step 2: Deproteinization
Deproteinization experiments were performed in order to determine the quantitative effect of different conditions for protein removal. For deproteinization (DP) investigations, a larger amount of demineralized pupal exuviae were previously prepared. This was performed in a 3 L-shaking flask with 200 g grinded pupal exuviae suspended in 800 mL 1 M formic acid incubated for 3 h at room temperature (RT) and a shaking frequency of 120 rpm. It has to be stated that the conditions slightly differ from those investigated in the demineralization study but are a result of few adaptions which did not lead to significant altered DME. However, biomass was afterwards separated by centrifugation (4696 g, 10 min) and subsequently washed with 1.8 L water. After another centrifugation step, the demineralized biomass was dried overnight at 105 °C.
DP was performed in a parallel reaction system (Tornado™IS6, R. B. Radley Co. Ltd, UK). 50 mL of the sodium hydroxide (NaOH) solution were heated in a 50 mL reaction vessel to the temperature fixed before. Reached the desired temperature, 5 g of demineralized pupal exuviae were added and the suspension stirred (300 rpm). Reaction was finished after the predefined incubation time by cooling on ice. The deproteinized material was separated with a glass funnel equipped with an integrated filter membrane (pore size 100–150 µm) under vacuum. Subsequent washing of the biomass with hot water (~ 250 mL) removed dyes and adsorbed proteins. The product was dried in an oven at 105 °C overnight and further analyzed concerning the amino acid content. The amino acid content was utilized to determine the protein content, and deproteinization efficiency (DPE) was calculated according to the following equation:
whereas PDep and PDem are the protein content in percentage after and before the reaction, respectively. The different conditions applied for the approaches and the certain results are provided in Table 1.
Temperature (°C), NaOH concentration (mol/L) and incubation time (h) were varied in randomized experiments in order to identify the most relevant deproteinization parameters. Response surface methodology (RSM) and linear regression were performed with Design-Expert® (Version 7.1.5, Stat-Ease Inc. Minneapolis, USA). Results were analyzed with the statistical software to determine the optimum conditions for the DP process and to assess validity and relevance of each parameters for demineralization efficiency.
Step 3: Bleaching
According to the results obtained by the evaluation of the DP experiments and to provide a sufficient amount of raw material for bleaching trials, a larger amount of demineralized pupal exuviae was subjected to DP at previously determined suitable conditions. Hence, 75 g of demineralized pupal exuviae were incubated for 2 h at 90 °C in 2.5 M NaOH at a liquid/solid-ratio of 10 and stirred at 500 rpm in a stainless-steel reactor (Versoclave, Büchi, Germany). Afterwards, supernatant was removed via filtration, the residue washed with 4 L 80 °C hot water and the deproteinized pupal exuviae subsequently dried at 105 °C.
Bleaching was carried out with agents and protocols identified in other studies originally applied for bleaching of cellulose or chitin derived by crab shells or insects. The individual approaches and the respective conditions for bleaching are listed in Table 2.
All experiments were carried out in duplicates and the bleaching solutions were separated from the biomass by centrifugation for 5 min at RT and 4696 g after the bleaching process. The samples were additionally washed with 250 mL of water at 80 °C (methods 1 and 2) or 40 °C (method 3). The wash solution was removed by further centrifugation under the already mentioned conditions. Subsequently, the bleached insect chitin was dried overnight at 105 °C.
Method 1
For bleaching experiments I–VI (Table 2), 0.5 g of the insect chitin were weighed into a 50 mL glass reactor vessel and incubated with 10 mL of the bleaching solution for 60 min (except experiment IV 90 min) at 200 rpm. The bleaching reactions were carried out in the parallel synthesis system (Tornado™, Radleys) which was also used for DP. Experiment IV had to be finished after five min due to strong foaming. The sample was washed and dried under the abovementioned conditions.
Method 2
The bleaching with solvent mixtures (experiments VII–VIII, cf. Table 2) was carried out in 15 mL reaction vessels in a table shaker. For this purpose, 0.5 g of sample were weighed into a vessel and these were each filled with 10 mL of bleaching solution. The incubation took place at RT for 2 h (experiment VII) or 1 h (experiment VIII), at 500 rpm.
Method 3
As an alternative to the bleaching with chemicals, the use of laccase was attempted to remove pigments from insect chitin. Laccase can cleave lignin [34], thus it was applied to unbleached chitin due to the structural similarity between monomeric lignin constituents and the catecholic compounds. In these experiments (IX and X), a batch was carried out only with laccase and citrate buffer (pH 5), and an approach with additional 3 mM ABTS 2,2′-azino-bis-(3-ethylbenzthiazolin-6-sulfonic acid). ABTS is a diammonium salt which supports the electron transfer of the oxidation as a mediator. Experiments were carried out in 100 mL shaking flasks in the shaker. 1 g of insect chitin was added to a flask with 20 mL of citrate buffer with or without ABTS and 0.42 mg of laccase (5.6 kU/g measured with ABTS). The batches were incubated at room temperature for 24 h, at 160 rpm (Table 2).
Bleaching Evaluation
In order to quantitatively assess the bleaching effect of the different treatments, the bleached chitin was colorimetrically measured in a spectrophotometer. The spectrophotometer measures the reflectance values by illuminating the surface of the sample over the entire spectrum of visible light. A pure white standard with 100% reflection and a black one with 0% reflection were used for calibration.
The CIELab system is a colour space defined by the International Commission on Illumination (French: Commission Internationale de l’Eclairage, CIE) in 1976. The internationally used CIELab colour space was utilized to relate reflectance spectra to colour and evaluate differences among samples. Using the device-independent 3D colour model, colour differences are determined numerically. CIELab describes optical colour perception in a three-dimensional space and divides it into two colour axes (red-green and blue-yellow) and one lightness axis (white-black) [http://cie.co.at/]. The lightness axis has a scale of 0–100, where 0 means pure black and 100 means pure white. Within this colour space, each colour is represented by a colour point with L*, a* and b* as coordinates. The colour difference between a bleached sample and a reference sample can be measured as the distance between their respective colour points. With this method, the increase in lightness due to the bleaching treatment and, thus, the bleaching effect can be determined.
Preparation of the Bleached Samples
The bleached insect chitin, previously dried and ground, was pressed into tablet-shaped discs (Ø 13 mm) using a laboratory press. Approximatively 200 mg of bleached chitin was transferred into the pressing device and pressed at 10 bar. The pressed pellets were then measured in the spectrophotometer and evaluated according to the lightness axis of the CIELab colour space.
Colorimetric Measurement of the Bleached Samples Using a Spectrophotometer
For the quantitative evaluation of the bleaching effect, chitin samples were measured colorimetrically using a spectrophotometer. This determines the coordinates in the 3D CIELab colour space through remission and transmission measurement via double-beam spectrophotometry.
First, unbleached insect chitin was measured as a standard and its colour was converted into coordinates. The bleached samples were then measured, also converted, and the distance to the coordinates of the standard was generated as difference (D) on the individual axes (DL*, Da*, Db*). The values of lightness distances (DL*) were used to assess the bleaching effect. Since the bleaching tests involved the brightening of the samples, the absolute lightness values L* were defined as a significant criterion for the evaluation of the bleaching effect. The higher the L* value, the brighter the sample and the more effective the bleaching treatment. Based on the L* values of the purified insect chitin as a reference sample (L* standard), the bleaching effect was calculated as percentage increase in lightness, using the following equation:
Results and Discussion
Pupal exuviae arose from the transformation of the pupae in H. illucens imago. A major challenge of the chitin purification thereof is the sclerotized structure of the cuticles, meaning the cross-linking of proteins with catechol derivatives and chitin, resulting in water extrusion, hardening and browning of the cuticle [35, 36]. The three consecutive steps of the chitin purification process from pupal exuviae were investigated: demineralization (step 1), deproteinization (step 2) and bleaching (step 3).
Characterization of the Raw Material
The metal ion contents of the pupal exuviae fresh mass were as follows: Ca2+ 40 mg/kg, Mg2+ 5 mg/kg, Mn2+ 0.8 mg/kg, Fe2+/3+ 0.15 mg/kg, Zn2+ 0.05 mg/kg, Cu2+ 8 µg/kg, Ni2+ 4 µg/kg. The pupal exuviae have a moisture content of 8.7%, and an ash content of 15.8% on a wet mass base. Lipid content was determined to be 1.2% related to wet mass. Khayrova et al. [37] have determined the ash and lipid content of H. illucens pupal exuviae, obtaining 10.5% and 8.9% respectively. At the best of our knowledge, there is no other work in the literature with which to compare our data. In most cases, the composition of larvae or prepupae of H. illucens, the stages most commonly intended for animal feed production, is determined.
Demineralization as the first step of purification
Previous investigations identified the major amount of the ash in the Black soldier fly as calcium and magnesium salts (not shown), whereby the main counter anion is carbonate [38]. The application of an acid results in the degradation of the carbonates and the formation of carbon dioxide which is the driving force of the reaction. Various acids were investigated at different temperatures concerning the demineralization efficiency, the results are reported in Fig. 1.
Treatment with acids was mandatory as indicated by the non-significant demineralization of pupal exuviae resulted from incubation with water at both 40 and 70 °C. The solubility of calcium carbonate (14 mg/L at 20 °C) (GESTIS substance database, 2020, https://gestis-database.dguv.de/data?name=001650) is too low to allow its removal with water only. Furthermore, calcium carbonate is an “inverse solubility salt” whose solubility decreases with increasing temperature [39].
At both temperatures, sulfuric acid and phosphoric acid showed the lowest DME, resulting in residual ash contents > 8% (Fig. 1). The use of phosphoric acid leads to the formation of heavily soluble calcium phosphates at slightly low pH values and an inverse solubility as already described for calcium carbonate [39]. The application of sulphuric acid at the given pH value leads to the formation of calcium salts with low solubility remaining in the biomass. What has just been said also applies to the magnesium salts. Hence both phosphoric and sulfuric acid are not suitable for demineralization of H. illucens pupal exuviae.
The use of nitric, hydrochloric, acetic and formic acids resulted in a much lower mineral content (1.6–5.2%) at both temperatures, with the highest DME being achieved treating with both 0.5 M formic acid or hydrochloric acid (Fig. 1). The salts formed as a result of the use of these acids (nitrates, chlorides, acetates and formates) have a higher solubility than calcium phosphate or sulphate. Comparing the mineral content after the 40 °C and 70 °C treatments with all acids, it is shown that the treatment at 70 °C did not result in the removal of more minerals as could be expected. With sulfuric and acetic acid only the DME was slightly higher at 70 °C than 40 °C.
Since it showed the highest DME at both 40 °C and 70 °C, formic acid was the acid of choice for DM of pupal exuviae.
These results are in line with studies on DM of shrimp shells with organic acids, such as lactic or acetic acid, reporting that the DME was comparable to that obtained using hydrochloric acid [40, 41]. Furthermore, the costs of demineralisation can be reduced by using organic acids [40] and salts of formic acid exhibit lower corrosiveness than mineral acids, which can reduce material wear on an industrial scale.
Formic acid has already been used for DM of H. illucens larval exoskeleton, obtaining a DME of 84–86% [20]. Similar values were achieved by Zhou et al. [42] using natural deep eutectic solvents for DM of prepupae. Khayrova et al. [43] and Smets et al. [44] performed DM of H. illucens larvae, prepupae and pupae with hydrochloric acid, but DME of these treatments was not been measured.
Investigation of the Deproteinization as Second Step of Chitin Purification
As is the case for chitin purification from crab shells, proteins are the second compound to be separated after demineralization in a deproteinization step. The protein content of samples was deduced from the mass sum of all amino acids quantified.
The amino acids content of raw pupal exuviae was 317.4 ± 30.9 mg/g, so the protein content of the pupal exuviae biomass was calculated to 31.7 ± 3.1% (w/w). This protein content is slightly lower than in the exoskeleton of other insects. For instance, exoskeleton of honeybees (Apis mellifera) contains around 40% proteins [45]. Cocoons of the fungus gnat (Rhynchosciara americana) have 38% protein [46], while cocoons of silkworm (Bombyx mori) have 48% proteins [47].
Deproteinization was carried out with the demineralized pupal exuviae. The deproteinization efficiency (DPE) was calculated for all the experiments. Results are reported in Table 3. The highest DPE resulted from incubation with 1.25 M NaOH solution for 4 h at 90 °C (experiment 2), during which 96% of the proteins was removed from the sample. The use of NaOH leads to partial or total hydrolysis of the peptide bonds, whereby the extent of the hydrolysis increases with the incubation temperature and alkali concentration. Indeed, the DPE raised sharply as the temperature was raised. With a temperature of 90 °C, the DPE was higher than 79% independent of incubation time and NaOH molarity.
Even incubating for 4 h, the lowest NaOH concentration (0.5 M) and a temperature of 70 °C (experiment 14), resulted in a minimal DPE of 20%. The DPE raised to 79% by increasing only the NaOH concentration to 1.25 M (experiment 3).
As already presumed by a superficial investigation of the results, temperature and NaOH concentration are significant parameters for DPE (p < 0.005). The dependency of the DPE in function of both parameters is illustrated in Fig. 2. The incubation time has lower significance. Thus, a linear model with two main effects was generated:
The F-value of 23.3 implies that the model is valid. Independent from the incubation time and according to the model, 90 °C and 2 M should result in a maximum DPE. Although providing a valid model, a R2 of 0.78 indicates that there is significant proportion of variation in the data which cannot be reasoned by the model. It has to be further stated that the optimum temperature and NaOH concentration are at the upper limit of the range applied for investigation. This implies, that higher values would be more efficient. Nevertheless, 100 °C is the limit for the water temperature conducting the deproteinization with common equipment and 2M NaOH is a suitable concentration to deproteinize the samples in an appropriate time span.
A quantitative evaluation of the DPE of insect biomass was only performed by few researchers. The DPE achieved within out treatment was confirmed by other studies: Kim and coworkers [24, 48] applied DP conditions similar to ours (1.25M NaOH for 3 h at 95 °C) on Musca domestica pupae and Gryllus bimaculatus adults achieving 87% DPE. A DPE of 97% was achieved by Zhou et al. [42] using natural deep eutectic solvents on H. illucens prepupae.
It has to be stated that the deproteinization at alkaline conditions and elevated temperatures commonly results in a partial deacetylation of the chitin as approved by Pires et al. [49]. However, the authors reported a deacetylation of about 13% of the acetyl groups at similar conditions as provided in the present study.
The use of NaOH is also accompanied by the solubilization of catechols that are incorporated into the insect carapaces due to the sclerotization process. Part of these catechols is known to be tightly bound to chitin [50] and they are not removed by the DP conditions applied. Thus, the use of bleaching reagents in a separate step is necessary to remove the residual coloration of the biomass. In the cocoons of the tobacco hawkmoth (Manduca sexta), 4% of catechols is present after sclerotization. By the time the adult hawkmoths hatch from the cocoons, the proportion of catechol derivatives increases up to 17% [51]. A similar catechol content can be expected for the H. illucens breeding waste.
Bleaching as the Final Process Step
The bleaching treatment was applied at the end of the purification process, to increase chitin lightness and purification degree by removing redox-sensitive and colour-intensive contaminants, mainly catecholic compounds. All bleaching experiments were carried out with the unbleached insect chitin, which was first demineralized and deproteinized with the process conditions identified to be optimal. The images of the resulting bleached samples are shown in Fig. 3. Bleaching effects of the different treatments were determined by colorimetric measurement using a spectrophotometer. The lightness values (L*) of bleached samples and the unbleached insect chitin as standard were first determined. With the knowledge of the L* value of the standard chitin (L* = 47.9), the DL* of each bleached sample (i.e. the difference of lightness between the standard and the bleached samples) and, consequently, the bleaching effect of the respective treatment were calculated. Lightness and bleaching effect values of each treatment are shown in Fig. 4. The best result was achieved using NaOCl solution, which led to an increase in L* by about 38, compared to the unbleached chitin, corresponding to a bleaching effect of 66.5%. The H2O2-containing solutions also led to very good bleaching. The use of H2O2 with NaOH resulted in a significantly higher bleaching effect (62.5%) than the use of H2O2 alone or with MgSO4 additive without lye. This makes clear that alkaline-soluble cleavage products are formed during oxidative bleaching [52]. These products remain in solution through the NaOH and can be removed during the separation of the solution. Less effective (7.7–13.9% bleaching effect) were reducing agents and solvent mixtures (experiments I, V, VII, VIII). The enzymatic approaches could not lead to a significant lightening. Only a bleaching effect of 0.3% was observed using laccase. The use of ABTS as mediator for laccase even resulted in a slight, reddish colouration of the solid based on a colour change from green to red when incubating the ABTS solution with laccase, citrate buffer and chitin. This dyeing was transferred to the solid and could not be alleviated by the washing step following the incubation.
Due to the environmental risk of chlorine-containing bleaching agents, it is desirable to identify a chlorine-free alternative to the bleaching of insect chitin. For instance, a treatment with potassium permanganate (KMnO4) and oxalic acid (C2H2O4) was proposed by Wasko et al. [53]. The use of 1% KMnO4 for 1 h at room temperature followed by 4% C2H2O4 for 1 h at 60 °C for bleaching of H. illucens adults and pupal exuviae chitin led to a white–gray product [53]. According to our results, the use of H2O2 can also be a valid alternative to chlorine-containing compounds, being its bleaching effect almost equal to that of NaOCl. Furthermore, combining bleaching with deproteinization using a mixture of H2O2 and NaOH, as done in the bleaching experiment IV, could be considered. The percentage used of NaOH (1.8%) corresponds to a concentration of about 0.5M. According to the results of our deproteinization experiments, the use of 0.5M NaOH at high temperature results in a DPE of 80%. Thus, a combination of H2O2 and an increased NaOH concentration could lead to a highly efficiency deproteinization and bleaching.
A comparison with literature values is challenging and not very significant, because of different evaluation methods and chitin sources. Crustacean shells, the major chitin source, has a faint red coloration which is mainly due to non-covalently bound carotenes [54] not contaminated with cross-linked catechols as in the exuviae of insects. However, some “lessons” can be learnt: Kaya et al. [55] proposed the use of a bleaching treatment with NaOCl as the first step in the purification process of chitin from crustacean, to reduce the time required for subsequent demineralization and deproteinization. This option could also be investigated for chitin isolation from insects.
Degree of Purification Within Processing of the Pupal Exuviae
In the three purification steps DM, DP and bleaching, different components of the sample material are removed and the chitin is enriched. To evaluate the purification, a wet chemical method (ADF, ADL, ADF–ADL) was performed after each step to determine the respective chitin content and to assess the purification. Chitin purity and related data during purification are shown in Table 3.
The results indicated a total loss of 83.5% biomass after the three extraction steps, with the highest losses occurring after both DM and DP to an equal extent. The lower loss in bleaching than with DM and DP was due to the proportionally lower presence of chromophores. The original biomass contained approx. 21.4% minerals and 31.7% proteins. The catechols were measured as ADL with a proportion of almost 13.0%.
Furthermore, it can be seen that the ADF was enriched by DM and DP from originally 32.7% to 90.8% by removing minerals and proteins. Considering the values of ADL, an increase of 5.5% (from 12.6 to 18.1%) occurred after DM compared to the raw sample (Table 3). Just like the ADF, it was initially enriched when the minerals were removed. However, a decrease to 11.4% was obtained by DP using NaOH. This can be explained by the fact that the splitting of the peptide bonds by means of NaOH also causes a part of the catechols to be dissolved and removed, indicated by the browning of the DP solution. The further decline of the ADL value after bleaching supports the assumption that the ADL relates to the catechol content of the insect cuticle. The aim of bleaching was to separate or disintegrate the catechols leading to darkening. The effectiveness of this treatment is illustrated by the lightening of the samples and an ADL value of only 4.2% after bleaching.
In order to evaluate the accumulation of chitin during purification, the values of ADF-ADL could be considered. Here, a stepwise increase through the individual purification steps can be seen. Starting from 20.0% in the dried raw sample, the value was increased to 84.7% after DM, DP and bleaching (Table 3). This result shows that the methods applied can be successfully used for the recovery and purification of chitin from insect remains of H. illucens. The chitin content of our final product can be assumed as a degree of purity of the chitin obtained from H. illucens pupal exuviae. A purity of 84.7% is in accordance with the average value (82.5%) obtained by Zhou et al. [42] purifying chitin from H. illucens prepupae with natural deep eutectic solvents. A slightly higher purity (93%) was achieved by chemical purification from Bombyx eri larvae using HCl and NaOH for DM and DP, respectively, without performing bleaching [56].
A biomass retention of 16.5% containing 84.7% chitin corresponds to a chitin yield of 13.9%, referring to the whole insect biomass applied for the extraction. This value is comparable to the average yield of 5–15% chitin obtained in most cases from different insect species [13]. Chitin has been purified from H. illucens pupal exuviae only by Brigode et al. [21] and Wang et al. [27] with a yield of 25 and 14%, respectively, using HCl for DM and NaOH for DP. Recently, also Złotko et al. [57] reported the isolation of chitin from pupal exuviae of this insect, using the same chemicals for DM and DP, but achieving a much lower chitin yield: 8% without bleaching, and 6–7% applying different discolouration treatments. Nevertheless, it should be noted that there is no uniformity in the calculation of the chitin yield, as it refers sometimes to the total biomass retained at the end of the extraction process, and sometimes to the chitin content of this final biomass obtained.
However, chitin enrichment is accompanied by loss of the biopolymer during the purification processes. On the one hand, this is done by filtration or sample transfer. On the other hand, a partial degradation of the chitin can also be assumed. From the initial value of the chitin content of 20.0%, the yield of biomass, the total masses and the chitin content after purification (84.7%), the loss of chitin amounts to 27.6% and is thus within an acceptable range.
Spectroscopic Characterization and Assessment of the Quality of Pupal Exuviae Chitin
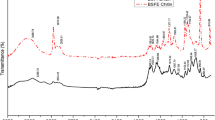
Fourier-transform Infrared spectroscopy (FT-IR) is a simple and powerful analytical technique to determine the functional groups present in chitin. The most significant bands of Hermetia illucens-derived chitin occur at wave numbers of 1310 cm−1 (CN stretching, amide III), 1560 cm−1 (NH bending, amide II), 1623 and 1655 cm−1 (CO stretching, amide I), 3105 cm−1 (NH symmetric stretching), 3255 cm−1 (NH asymmetric stretching) and 3440 cm−1 (OH stretching) (Fig. 5A, see commercial chitin for reference) [13, 58]. The two separate bands at 1623 cm−1 and 1655 cm−1 indicate the presence of α-chitin, which is related to the occurrence of the intermolecular hydrogen band –CO⋯HN– and –CO⋯HOCH2 [59].
Spectroscopic characterization of purified α-chitin from pupal exuviae. A shows the FT-IR spectra of the commercial P. borealis chitin (red) and the pupal exuviae chitin (black). B illustrates the XRD diffractogram of the pupal exuviae chitin and a theoretical pattern of the crystal data of α-chitin. C. shows the ss-NMR of the purified chitin. The arrow indicates the presence of proteins or/and catecholic compounds. (Color figure online)
Powder X-ray diffraction shows broad reflections at 2θ values of 9° (020), 13° (021), 20° (110), 21° (120), 23° (130), 26° (031) indicating the occurrence of the lattice planes (020), (021), (110), (120) (130) and (031) of the crystal of α-chitin (see Fig. 5B) [60]. The theoretical X-ray diffraction pattern was published by Sikorski et al. [60] and emphasizes the relevant signals derived from the different planes. The crystallinity index of the chitin was calculated to be CI110 = 74%. This value is comparable to the common crystallinity indices of 72–83% in different α-chitin sources [61,62,63].
13C solid-state nuclear magnetic resonance (ss-NMR) is able to assess the quality of chitin such as the degree of acetylation (DA), polymorphic forms and impurities such as proteins or catecholic compounds [64,65,66]. The ss-NMR of the bleached chitin extracted from pupal exuviae, shows peaks at 173.1 (C7), 104.3 (C1), 83.2 (C4), 75.8 (C5), 73.5 (C3), 60.9 (C6), 55.2 (C2), 22.9 (C8) ppm (see Fig. 5C), which provides all typical peaks of chitin [67]. An additional peak at 30 ppm (s. Fig. 5C, see notation) indicates the presence of proteins or/and catecholic compounds [62, 65, 68, 69]. This result may provide further support for the hypothesis that catecholic compound are still present in the purified chitin after bleaching. The DA of the purified chitin is found to be 96%. In literature, insect- and crustacean-derived α-chitin commonly exhibited DAs of 90–98% [65, 68, 70].
Conclusions
This investigation provides an optimization of chitin extraction from side streams of insect farming. Pupal exuviae of the widely bred fly species H. illucens were used, being them a readily available waste biomass rich in chitin easy to collect from the insect-breeding facilities. Steps of the extraction process were carried out under different conditions in order to assess the optimal parameters.
DM was optimized concerning acid applied and temperature. Formic and hydrochloric acid were the most effective, particularly formic acid gave the best results at both tested temperatures. This encourages the use of organic acids instead of the most applied inorganic acids in a frame of a greater environmental sustainability.
Efficiency of DP was investigated in relation to temperature, molarity and duration, highlighting the significance of temperature and alkali molarity as parameters for an efficient protein removal. The optimized DP procedure successfully removed proteins from the insect sample. At the same time, the use of NaOH leads to a slight or marginal deacetylation of the final chitin [71]. In comparison, other studies reported about 23% deacetylation after treating chitin with 2% NaOH for 0.5 h at 60 °C [72].
The bleaching experimental series shows that oxidative agents lead to a significant lightening of the insect chitin, especially solutions with NaOCl and H2O2. Although the bleaching effect is slightly higher using NaOCl, the use of H2O2 can be a viable alternative to reduce the environmental risk of chlorine-containing compounds while still maintaining a high bleaching effect. The possibility of bleaching and deproteinizing at the same time using a combination of H2O2 and NaOH can be also considered, thus saving time and energy.
Appling the optimized extraction method, a chitin with around 85% purity was obtained from H. illucens pupal exuviae with a yield of 14% related to the original insect biomass. This is in line with average yields and purity of chitins generally obtained from insects. Particularly, the purification degree of chitin extracted from pupal exuviae is similar to that experimentally determined of commercial chitin from fishery waste (unpublished data). The spectroscopic investigation of the pupal exuviae chitin indicates that traces of protein/catecholic compounds are present in the purified biopolymer. The extracted α-chitin has a DA of 96% and a CI of 74%.
Currently, insect-based chitin is produced only on a laboratory scale. This study provides a starting point, on one hand, for the scale-up of chitin purification processes to exploit waste biomass generated from the insect farms. On the other hand, it provides the basis for further research activities fully unfolding the potential of the chitin and its derivatives isolated from insects. This was already confirmed by researchers applying chitosan obtained from H. illucens pupal exuviae for advanced applications, such as 3D films [73].
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Elieh-Ali-Komi, D., Hamblin, M.R.: Chitin and chitosan: production and application of versatile biomedical nanomaterials. Int. J. Adv. Res. 4(3), 411–427 (2016)
Manjula, K., Podile, A.R.: Chitin-supplemented formulations improve biocontrol and plant growth promoting efficiency of Bacillus subtilis AF 1. Canad. J. Microbiol. 47(7), 618–625 (2001). https://doi.org/10.1139/w01-057
Wang, M., Chen, L.J., Ni, J., Weng, J., Yue, C.Y.: Manufacture and evaluation of bioactive and biodegradable materials and scaffolds for tissue engineering. J. Mater. Sci. Mater. Med. 12(10), 855–860 (2001). https://doi.org/10.1023/A:1012899318688
Felse, P.A., Panda, T.: Studies on applications of chitin and its derivatives. Bioprocess Eng. 20(6), 505–512 (1999). https://doi.org/10.1007/s004490050622
Yeul, V.S., Rayalu, S.S.: Unprecedented chitin and chitosan: a chemical overview. J. Polym. Environ. 21(2), 606–614 (2012). https://doi.org/10.1007/s10924-012-0458-x
Bolat, Y., Bilgin, Ş, Günlü, A., Izci, L., Koca, S.B., Çetinkaya, S., Koca, H.U.: Chitin-chitosan yield of freshwater crab (Potamon potamios, Olivier 1804) shell. Pak. Vet. J. 30(4), 227–231 (2010)
Kurita, K.: Chitin and chitosan: functional biopolymers from marine crustaceans. Mar. Biotechnol. (NY) 8(3), 203–226 (2006). https://doi.org/10.1007/s10126-005-0097-5
Jardine, A., Sayed, S.: Valorisation of chitinous biomass for antimicrobial applications. Pure Appl. Chem. 90(2), 293–304 (2018). https://doi.org/10.1515/pac-2017-0707
Gillett, R.: Global Study of Shrimp Fisheries, vol 475. FAO Fisheries Technical Paper. Food and Agriculture Organization of the United Nations, Rome (2008)
Pittman, S.J., McAlpine, C.A.: Movements of marine fish and decapod crustaceans: process, theory and application. Adv. Mar. Biol. 44(1), 205–294 (2003)
White, S.A., Farina, P.R., Fulton, I.: Production and isolation of chitosan from Mucor rouxii. Appl. Environ. Microbiol. 38(2), 323–328 (1979)
Abo Elsoud, M.M., El Kady, E.M.: Current trends in fungal biosynthesis of chitin and chitosan. Bull. Natl. Res. Centre 43(1), 59 (2019). https://doi.org/10.1186/s42269-019-0105-y
Hahn, T., Tafi, E., Paul, A., Salvia, R., Falabella, P., Zibek, S.: Current state of chitin purification and chitosan production from insects. J. Chem. Technol. Biotechnol. 95(11), 2775–2795 (2020). https://doi.org/10.1002/jctb.6533
Fowles, T.M., Nansen, C.: Insect-based bioconversion: value from food waste. In: Närvänen, E., Mesiranta, N., Mattila, M., Heikkinen, A. (eds.) Food Waste Management: Solving the Wicked Problem, pp. 321–346. Springer, Cham (2020). https://doi.org/10.1007/978-3-030-20561-4_12
van Huis, A.: Potential of insects as food and feed in assuring food security. Annu Rev Entomol 58, 563–583 (2013). https://doi.org/10.1146/annurev-ento-120811-153704
Jucker, C., Lupi, D., Moore, C.D., Leonardi, M.G., Savoldelli, S.: Nutrient recapture from insect farm waste: bioconversion with Hermetia illucens (L) (Diptera: Stratiomyidae). Sustainability 12(1), 1–14 (2020). https://doi.org/10.3390/su12010362
Tomberlin, J.K., Sheppard, D.C., Joyce, J.A.: Selected life-history traits of black soldier flies (Diptera : Stratiomyidae) reared on three artificial diets. Ann. Entomol. Soc. Am. 95(3), 379–386 (2002). https://doi.org/10.1603/0013-8746(2002)095[0379:slhtob]2.0.co;2
Hahn, T., Roth, A., Febel, E., Fijalkowska, M., Schmitt, E., Arsiwalla, T., Zibek, S.: New methods for high-accuracy insect chitin measurement. J. Sci. Food Agric. 98(13), 5069–5073 (2018). https://doi.org/10.1002/jsfa.9044
Schmitt, E., de Vries, W.: Potential benefits of using Hermetia illucens frass as a soil amendment on food production and for environmental impact reduction. Curr. Opin. Green Sustain. Chem. 25, 100335 (2020). https://doi.org/10.1016/j.cogsc.2020.03.005
Hahn, T., Roth, A., Ji, R., Schmitt, E., Zibek, S.: Chitosan production with larval exoskeletons derived from the insect protein production. J. Biotechnol. 310, 62–67 (2020)
Brigode, C., Hobbi, P., Jafari, H., Verwilghen, F., Baeten, E., Shavandi, A.: Isolation and physicochemical properties of chitin polymer from insect farm side stream as a new source of renewable biopolymer. J. Clean. Prod. (2020). https://doi.org/10.1016/j.jclepro.2020.122924
Badawy, R.M., Mohamed, H.I.: Chitin extration, composition of different six insect species and their comparable characteristics with that of the shrimp. J. Am. Sci. 11(6), 127–134 (2015)
Kaya, M., Bagriacik, N., Seyyar, O., Baran, T.: Comparison of chitin structures derived from three common wasp species (Vespa crabro LINNAEUS, 1758, Vespa orientalis LINNAEUS, 1771 and Vespula germanica (FABRICIUS, 1793)). Arch. Insect Biochem. Physiol. 89(4), 204–217 (2015). https://doi.org/10.1002/arch.21237
Kim, M.-W., Song, Y.-S., Han, Y.S., Jo, Y.H., Choi, M.H., Park, Y.-K., Kang, S.H., Kim, S.-A., Choi, C., Jung, W.-J.: Production of chitin and chitosan from the exoskeleton of adult two-spotted field crickets (Gryllus bimaculatus). Entomol. Res. 47(5), 279–285 (2017). https://doi.org/10.1111/1748-5967.12239
Ibitoye, E.B., Lokman, I.H., Hezmee, M.N.M., Goh, Y.M., Zuki, A.B.Z., Jimoh, A.A.: Extraction and physicochemical characterization of chitin and chitosan isolated from house cricket. Biomed. Mater. 13(2), 1–12 (2018). https://doi.org/10.1088/1748-605X/aa9dde
Shin, C.-S., Kim, D.-Y., Shin, W.-S.: Characterization of chitosan extracted from Mealworm Beetle (Tenebrio molitor, Zophobas morio) and Rhinoceros Beetle (Allomyrina dichotoma) and their antibacterial activities. Int. J. Biol. Macromol. 125, 72–77 (2019). https://doi.org/10.1016/j.ijbiomac.2018.11.242
Wang, H., Rehman, K.U., Feng, W., Yang, D., Rehman, R.U., Cai, M., Zhang, J., Yu, Z., Zheng, L.: Physicochemical structure of chitin in the developing stages of black soldier fly. Int. J. Biol. Macromol. 149, 901–907 (2020). https://doi.org/10.1016/j.ijbiomac.2020.01.293
Matissek, R., Steiner, G., Fischer, M.: Fette und Fettbegleitstoffe. In: Lebensmittelanalytik, pp. 29–83. Springer, Berlin Heidelberg (2010). https://doi.org/10.1007/978-3-540-92205-6_2
Urbat, F., Müller, P., Hildebrand, A., Wefers, D., Bunzel, M.: Comparison and optimization of different protein nitrogen quantitation and residual protein characterization methods in dietary fiber preparations. Front. Nutr. (2019). https://doi.org/10.3389/fnut.2019.00127
Kumirska, J., Czerwicka, M., Kaczyński, Z., Bychowska, A., Brzozowski, K., Thöming, J., Stepnowski, P.: Application of spectroscopic methods for structural analysis of chitin and chitosan. Mar. Drugs 8(5), 1567–1636 (2010). https://doi.org/10.3390/md8051567
Focher, B., Beltrame, P.L., Naggi, A., Torri, G.: Alkaline N-deacetylation of chitin enhanced by flash treatments. Reaction kinetics and structure modifications. Carbohyd. Polym. 12(4), 405–418 (1990). https://doi.org/10.1016/0144-8617(90)90090-F
Ottey, M.H., Vårum, K.M., Smidsrød, O.: Compositional heterogeneity of heterogeneously deacetylated chitosans. Carbohyd. Polym. 29(1), 17–24 (1996). https://doi.org/10.1016/0144-8617(95)00154-9
Kasaai, M.R.: Determination of the degree of N-acetylation for chitin and chitosan by various NMR spectroscopy techniques: a review. Carbohyd. Polym. 79(4), 801–810 (2010). https://doi.org/10.1016/j.carbpol.2009.10.051
Christopher, L.P., Yao, B., Ji, Y.: Lignin biodegradation with laccase-mediator systems. Front. Energy Res. (2014). https://doi.org/10.3389/fenrg.2014.00012
Gonil, P., Sajomsang, W.: Applications of magnetic resonance spectroscopy to chitin from insect cuticles. Int. J. Biol. Macromol. 51(4), 514–522 (2012). https://doi.org/10.1016/j.ijbiomac.2012.06.025
Hopkins, T.L., Kramer, K.J.: Insect cuticle sclerotization. Annu Rev Entomol 37, 273–302 (1992)
Khayrova, A., Lopatin, S., Varlamov, V.: Obtaining chitin/chitosan-melanin complexes from black soldier fly Hermetia Illucens. IOP Conf. Ser. Mater. Sci. Eng. (2020). https://doi.org/10.1088/1757-899x/809/1/012020
Barros-Cordeiro, K.B., Bao, S.N., Pujol-Luz, J.R.: Intra-puparial development of the black soldier-fly, Hermetia illucens. J. Insect Sci. 14, 83 (2014)
Bott, T.R.: Crystallisation and scale formation. In: Bott, T.R. (ed.) Fouling of heat exchangers, pp. 97–135. Elsevier, Amsterdam (1995). https://doi.org/10.1016/B978-044482186-7/50010-5
Mahmoud, N.S., Ghaly, A.E., Arab, F.: Unconventional approach for demineralization of deproteinized crustacean shells for chitin production. Am. J. Biochem. Biotechnol. 3(1), 1–9 (2007). https://doi.org/10.3844/ajbbsp.2007.1.9
Alewo, A., David, A., Isa, M., Rabiu, U.: Kinetics of demineralization of shrimp shell using lactic acid. Leonardo El. J. Pract. Technol. 13, 13–22 (2014)
Zhou, P., Li, J., Yan, T., Wang, X., Huang, J., Kuang, Z., Pan, M.: Selectivity of deproteinization and demineralization using natural deep eutectic solvents for production of insect chitin (Hermetia illucens). Carbohyd. Polym. 225, 1–9 (2019). https://doi.org/10.1016/j.carbpol.2019.115255
Khayrova, A., Lopatin, S., Varlamov, V.: Black soldier fly Hermetia illucens as a novel source of chitin and chitosan. Int. J. Sci. 8(04), 81–86 (2019). https://doi.org/10.18483/ijSci.2015
Smets, R., Verbinnen, B., Van De Voorde, I., Aerts, G., Claes, J., Van Der Borght, M.: Sequential extraction and characterisation of lipids, proteins, and chitin from black soldier fly (Hermetia illucens) larvae, prepupae, and pupae. Waste Biomass Valor. (2020). https://doi.org/10.1007/s12649-019-00924-2
Nemtsev, S.V., Zueva, O.Y., Khismatullin, M.R., Albulov, A.I., Varlamov, V.P.: Isolation of chitin and chitosan from honeybees. Appl Biochem Micro+ 40(1), 39–43 (2004)
Terra, W.R., de Bianchi, A.G.: Chemical composition of the cocoon of the fly, Rhynchosciara americana. Insect Biochem. 4(2), 173–183 (1974). https://doi.org/10.1016/0020-1790(74)90005-5
Rumpold, B.A., Schluter, O.K.: Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 57(5), 802–823 (2013). https://doi.org/10.1002/mnfr.201200735
Kim, M.-W., Han, Y.S., Jo, Y.H., Choi, M.H., Kang, S.H., Kim, S.A., Jung, W.J.: Extraction of chitin and chitosan from housefly, Musca domestica, pupa shells. Entomol. Res. 46(5), 324–328 (2016). https://doi.org/10.1111/1748-5967.12175
Pires, C.T.G.V.M.T., Vilela, J.A.P., Airoldi, C.: The effect of chitin alkaline deacetylation at different condition on particle properties. Procedia Chem. 9, 220–225 (2014). https://doi.org/10.1016/j.proche.2014.05.026
Xu, R., Huang, X., Hopkins, T.L., Kramer, K.J.: Catecholamine and histidyl protein cross-linked structures in sclerotized insect cuticle. Insect Biochem. Mol. 27(2), 101–108 (1997). https://doi.org/10.1016/S0965-1748(96)00083-5
Kramer, K.J., Hopkins, T.L., Schaefer, J.: Applications of solids NMR to the analysis of insect sclerotized structures. Insect Biochem. Mol. 25(10), 1067–1080 (1995). https://doi.org/10.1016/0965-1748(95)00053-4
Sun, R., Lu, Q., Sun, X.F.: Physico-chemical and thermal characterization of lignins from Caligonum monogoliacum and Tamarix spp. Polym. Degrad. Stabil. 72(2), 229–238 (2001). https://doi.org/10.1016/S0141-3910(01)00023-4
Wasko, A., Bulak, P., Polak-Berecka, M., Nowak, K., Polakowski, C., Bieganowski, A.: The first report of the physicochemical structure of chitin isolated from Hermetia illucens. Int. J. Biol. Macromol. 92, 316–320 (2016). https://doi.org/10.1016/j.ijbiomac.2016.07.038
Vilasoa-Martínez, M., Calaza-Ramos, C., López-Hernández, J., Lage-Yusty, M.A., Losada, P.P., Rodríguez-Bernaldo de Quirós, A.: Determination of vitamin E and carotenoid pigments by high performance liquid chromatography in shell of Chionoecetes opilio. Anal. Chim. Acta 617(1), 225–229 (2008). https://doi.org/10.1016/j.aca.2008.03.001
Kaya, M., Baran, T., Karaarslan, M.: A new method for fast chitin extraction from shells of crab, crayfish and shrimp. Nat. Prod. Res. 29(15), 1477–1480 (2015). https://doi.org/10.1080/14786419.2015.1026341
Huet, G., Hadad, C., Husson, E., Laclef, S., Lambertyn, V., Araya Farias, M., Jamaly, A., Courty, M., Alayoubi, R., Gosselin, I., Sarazin, C., Van Nhien, A.N.: Straightforward extraction and selective bioconversion of high purity chitin from Bombyx eri larva: toward an integrated insect biorefinery. Carbohydr. Polym. 228, 1–12 (2020). https://doi.org/10.1016/j.carbpol.2019.115382
Złotko, K., Waśko, A., Kamiński, D.M., Budziak-Wieczorek, I., Bulak, P., Bieganowski, A.: Isolation of chitin from black soldier fly (Hermetia illucens) and its usage to metal sorption. Polymers-Basel 13(5), 818 (2021)
Seoudi, R., Nada, A.M.A., Abd Elmongy, S., Hamed, S.S.: Fourier transform infrared spectroscopic and AC conductivity studies of chitin and its derivatives. J. Appl. Polym. Sci. 98(2), 936–943 (2005). https://doi.org/10.1002/app.22208
Focher, B., Naggi, A., Torri, G., Cosani, A., Terbojevich, M.: Structural differences between chitin polymorphs and their precipitates from solutions—evidence from CP-MAS 13C-NMR, FT-IR and FT-Raman spectroscopy. Carbohyd. Polym. 17(2), 97–102 (1992). https://doi.org/10.1016/0144-8617(92)90101-U
Sikorski, P., Hori, R., Wada, M.: Revisit of alpha-chitin crystal structure using high resolution X-ray diffraction data. Biomacromolecules 10(5), 1100–1105 (2009). https://doi.org/10.1021/bm801251e
Kaya, M., Sargin, I., Aylanc, V., Tomruk, M.N., Gevrek, S., Karatoprak, I., Colak, N., Sak, Y.G., Bulut, E.: Comparison of bovine serum albumin adsorption capacities of α-chitin isolated from an insect and β-chitin from cuttlebone. J. Ind. Eng. Chem. 38, 146–156 (2016). https://doi.org/10.1016/j.jiec.2016.04.015
Devi, R., Dhamodharan, R.: Pretreatment in hot glycerol for facile and green separation of chitin from prawn shell waste. ACS Sustain. Chem. Eng. 6(1), 846–853 (2018). https://doi.org/10.1021/acssuschemeng.7b03195
Cárdenas, G., Cabrera, G., Taboada, E., Miranda, S.P.: Chitin characterization by SEM, FTIR, XRD, and 13C cross polarization/mass angle spinning NMR. J. Appl. Polym. Sci. 93(4), 1876–1885 (2004). https://doi.org/10.1002/app.20647
King, C., Stein, R.S., Shamshina, J.L., Rogers, R.D.: Measuring the purity of chitin with a clean, quantitative solid-state NMR method. ACS Sustain. Chem. Eng. 5(9), 8011–8016 (2017). https://doi.org/10.1021/acssuschemeng.7b01589
Sajomsang, W., Gonil, P.: Preparation and characterization of α-chitin from cicada sloughs. Mater. Sci. Eng. C 30(3), 357–363 (2010). https://doi.org/10.1016/j.msec.2009.11.014
Kaya, M., Mujtaba, M., Ehrlich, H., Salaberria, A.M., Baran, T., Amemiya, C.T., Galli, R., Akyuz, L., Sargin, I., Labidi, J.: On chemistry of gamma-chitin. Carbohyd. Polym. 176, 177–186 (2017). https://doi.org/10.1016/j.carbpol.2017.08.076
Heux, L., Brugnerotto, J., Desbrières, J., Versali, M.F., Rinaudo, M.: Solid state NMR for determination of degree of acetylation of chitin and chitosan. Biomacromolecules 1(4), 746–751 (2000). https://doi.org/10.1021/bm000070y
Majtan, J., Bilikova, K., Markovic, O., Grof, J., Kogan, G., Simuth, J.: Isolation and characterization of chitin from bumblebee (Bombus terrestris). Int. J. Biol. Macromol. 40(3), 237–241 (2007). https://doi.org/10.1016/j.ijbiomac.2006.07.010
Richarz, R., Wüthrich, K.: Carbon-13 NMR chemical shifts of the common amino acid residues measured in aqueous solutions of the linear tetrapeptides H-Gly-Gly-X-L-Ala-OH. Biopolymers 17(9), 2133–2141 (1978). https://doi.org/10.1002/bip.1978.360170908
Van de Velde, K., Kiekens, P.: Structure analysis and degree of substitution of chitin, chitosan and dibutyrylchitin by FT-IR spectroscopy and solid state 13C NMR. Carbohyd. Polym. 58(4), 409–416 (2004). https://doi.org/10.1016/j.carbpol.2004.08.004
Xiaofei, H., Li, K., Xing, R., Liu, Q., Hu, L., Li, P.: The production of fully deacetylated chitosan by compression method. Egypt. J. Aquat. Res. (2016). https://doi.org/10.1016/j.ejar.2015.09.003
Mizami AMA, B. M. (2007) A new process of deproteinization of chitin from shrimp head waste. 8
Bilican, I., Pekdemir, S., Onses, M.S., Akyuz, L., Altuner, E.M., Koc-Bilican, B., Zang, L.-S., Mujtaba, M., Mulerčikas, P., Kaya, M.: Chitosan loses innate beneficial properties after being dissolved in acetic acid: supported by detailed molecular modeling. ACS Sustain. Chem. Eng. 8(49), 18083–18093 (2020). https://doi.org/10.1021/acssuschemeng.0c06373
Acknowledgements
We gratefully acknowledge ERA-IB, PTJ and BMBF for organization, administration and funding during the project ChitoTex (Grant Agree Number 031A567A) and the Carl Zeiss foundation for funding the project Chitinfluid (Grant Agree Number P2019-02-004). Additionally, we thank Dr. Michael Dyballa for the ss-NMR measurements and Jessica Bauhof and Prof. Dr. Thomas Sottmann for the X-ray diffraction measurements.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hahn, T., Tafi, E., von Seggern, N. et al. Purification of Chitin from Pupal Exuviae of the Black Soldier Fly. Waste Biomass Valor 13, 1993–2008 (2022). https://doi.org/10.1007/s12649-021-01645-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-021-01645-1