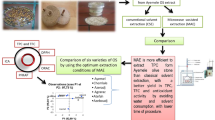
Abstract
Purpose
Maslinic and oleanolic acids are some of the valuable bioactive compounds present in olive pomace. The aim of this study is to investigate the direct use of wet olive pomace (without drying) for the extraction of these acids by taking advantage of the microwave technique and to determine the effect of extraction parameters on yield and quality.
Methods
The effects of microwave power (150–300 Watt), extraction time (4–20 min), and solvent to solid ratio (2.5–10) on extraction efficiency were examined. The extract further separated into maslinic and oleanolic acid fractions. The antioxidant activity and the antimicrobial effect of both extract and acid fractions were determined.
Results
Optimum conditions which provide the highest yield were found as 250 Watt, 12 min, and 10:1 for microwave power, time, and solvent to solid ratio, respectively. The total amount of maslinic and oleanolic acid obtained under optimum conditions was 26 ± 1.27 mg/g dry matter corresponding to 98.21% of the extract. The percentages of triterpenic acids present in the extract were 80% maslinic acid and 20% oleanolic acid. According to the DPPH (% inhibition 22.9 ± 0.8) and FRAP (263.1 ± 3.9 μM TE) results the maslinic acid fraction has shown the highest antioxidant activity. In addition, it was observed that the maslinic acid fraction had almost as much antimicrobial effect on the microorganisms as amoxicillin.
Conclusion
Microwave-assisted extraction could be an efficient way for the extraction of maslinic and oleanolic acids from wet olive pomace with minimal processing and high yield.
Graphic Abstract





Similar content being viewed by others
Data Availability
The online version contains supplementary material available at https://doi.org/10.6084/m9.figshare.14420702.v4
Code Availability
Not applicable.
References
Food and Agriculture Organization of the United Nations. FAOSTAT statistical database. [Rome]: FAO (2019)
Roig, A., Cayuela, M.L., Sanchez-Monedero, M.A.: An overview on olive mill wastes and their valorisation methods. Waste Manage. 26, 960–969 (2006). https://doi.org/10.1016/j.wasman.2005.07.024
Romero, C., Garcia, A., Medina, E., Ruiz-Mendez, M.V., Castro, A., Brenes, M.: Triterpenic acids in table olives. Food Chem. 118, 670–674 (2010). https://doi.org/10.1016/j.foodchem.2009.05.037
Rufino-Palomares, E.E., Reyes-Zurita, F.J., García-Salguero, L., Mokhtari, K., Medina, P.P., Lupiáñez, J.A., Peragón, J.: Maslinic acid, a triterpenic anti-tumoural agent, interferes with cytoskeleton protein expression in HT29 human colon-cancer cells. J. Proteomics. 83, 15–25 (2013). https://doi.org/10.1016/j.jprot.2013.02.031
Ghafoor, K.: Antioxidant properties of oleanolic acid from grape peel. Agro. Food Ind Hi-Tech. 25, 54–57 (2014)
Jamkhande, P.G., Pathan, S.K., Wadher, S.J.: In silico PASS analysis and determination of antimycobacterial, antifungal, and antioxidant efficacies of maslinic acid in an extract rich in pentacyclic triterpenoids. Int. J. Mycobacteriol. 5, 417–425 (2016). https://doi.org/10.1016/j.ijmyco.2016.06.020
Nur, N.M., Al-Jasabi, S.M.: Antioxidant properties of maslinic acid extracted from Plumeria rubra leaves. IJCRR 8, 20178–20183 (2017). https://doi.org/10.15520/ijcrr/2017/8/07/160
Özkan, G., Karacabey, E., Arslan, N., Odabasi, N.: Optimisation of microwave-assisted extraction of triterpenoic acids from olive mill waste using response surface methodology. Qual. Assur. Saf. Crop Foods. 9, 179–185 (2017). https://doi.org/10.3920/qas2015.0783
Xie, P., Huang, L., Zhang, C., Deng, Y., Wang, X., Cheng, J.: Enhanced extraction of hydroxytyrosol, maslinic acid and oleanolic acid from olive pomace: process parameters, kinetics and thermodynamics, and greenness assessment. Food Chem. 276, 662–674 (2019). https://doi.org/10.1016/j.foodchem.2018.10.079
Alexandraki, V., Georgalaki, M., Papadimitriou, K., Anastasiou, R., Zoumpopoulou, G., Chatzipavlidis, I., Papadelli, M., Vallis, N., Moschochoritis, K., Tsakalidou, E.: Determination of triterpenic acids in natural and alkaline-treated Greek table olives throught the fermentation process. LWT 5, 609–613 (2014). https://doi.org/10.1016/j.lwt.2014.04.005
Li, J., Zu, Y.-G., Fu, Y.J., Yang, Y.-C., Li, S.-M., Li, Z.-N., Wink, M.: Optimization of microwave-assisted extraction of triterpene saponins from defatted residue of yellow horn (Xanthoceras sorbifolia Bunge.) kernel and evaluation of its antioxidant activity. Innov. Food Sci. Emerg. Technol. 11, 637–643 (2010). https://doi.org/10.1016/j.ifset.2010.06.004
Medina, E., Romero, C., Brenes, M.: Residual olive paste as a source of phenolic compounds and triterpenic acids. Eur. J. Lipid Sci. Technol. 120, 1700368 (2018). https://doi.org/10.1002/ejlt.201700368
Fernandez-Pastor, I., Fernandez-Hernandez, A., Perez-Criado, S., Rivas, F., Martinez, A., Garcia-Granados, A., Parra, A.: Microwave-assisted extraction versus Soxhlet extraction to determine triterpene acids in olive skins. J. Sep. Sci. 40, 1209–1217 (2017). https://doi.org/10.1002/jssc.201601130
Garcia-Granados, A.: Process for the industrial recovery of oleanolic and maslinic acids contained in the olive milling byproducts, PCT. Int. Appl. WO 9804331 (1998)
Kuno, N., Shinohara, G.: Method for the preparation of oleanolic acid and/or maslinic acid. US Patent 6740778B2 (2004)
Amarni, F., Kadi, H.: Kinetics study of microwave-assisted solvent extraction of oil from olive cake using hexane: comparison with the conventional extraction. Innov. Food Sci. Emerg. Technol. 11, 322–327 (2010). https://doi.org/10.1016/j.ifset.2010.01.002
Yanık, D.K.: Alternative to traditional olive pomace oil extraction systems: microwave-assisted solvent extraction of oil from wet olive pomace. LWT 77, 45–51 (2017). https://doi.org/10.1016/j.lwt.2016.11.020
Pérez-Serradilla, J.A., Japon-Lujan, R., de Castro, M.L.: Simultaneous microwave-assisted solid–liquid extraction of polar and nonpolar compounds from alperujo. Anal. Chim. Acta 602, 82–88 (2007). https://doi.org/10.1016/j.aca.2007.09.008
Dontha, S., Kamurthy, H., Mantripragada, B.R.: Phytochemical characterization of active constituents from extracts of Ixora javanica D.C flowers. J. Chromatogr. Sep. Tech. 6, 294 (2015). https://doi.org/10.4172/2157-7064.1000294
Brand-Williams, W., Cuvelier, M.E., Berset, C.L.W.T.: Use of a free radical method to evaluate antioxidant activity. LWT 28, 25–30 (1995). https://doi.org/10.1016/s0023-6438(95)80008-5
Benzie, I.F., Strain, J.J.: The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 239, 70–76 (1996). https://doi.org/10.1006/abio.1996.0292
EUCAST: European Committee on Antimicrobial Susceptibility Testing breakpoint tables for interpretation of MICs and zone diameters. (2015)
Mandal, V., Mandal, S.: Design and performance evaluation of a microwave based low carbon yielding extraction technique for naturally occurring bioactive triterpenoid: oleanolic acid. Biochem. Eng. J. 50, 63–70 (2010). https://doi.org/10.1016/j.bej.2010.03.005
Ameer, K., Shahbaz, H.M., Kwon, J.-H.: Green extraction methods for polyphenols from plant matrices and their byproducts: a review. Compr. Rev. Food Sci. Food Saf. 16, 295–315 (2017). https://doi.org/10.1111/1541-4337.12253
Xiao, W., Han, L., Shi, B.: Microwave-assisted extraction of flavonoids from Radix Astragali. Sep. Purif. Technol. 62, 614–618 (2008). https://doi.org/10.1016/j.seppur.2008.03.025
Guo, Z., Jin, Q., Fan, G., Duan, Y., Qin, C., Wen, M.: Microwave-assisted extraction of effective constituents from a Chinese herbal medicine Radix puerariae. Anal Chim Acta. 436, 41–47 (2001). https://doi.org/10.1016/s0003-2670(01)00900-x
Fan, H., Yang, G.-Z., Zheng, T., Mei, Z.-N., Liu, X.-M., Chen, Y., Chen, S.: Chemical constituents with free-radical-scavenging activities from the stem of Microcos paniculata. Molecules 15, 5547–5560 (2010). https://doi.org/10.3390/molecules15085547
Çulhaoğlu, B., Hatipoğlu, S.D., Dönmez, A.A., Topçu, G.: Antioxidant and anticholinesterase activities of lupane triterpenoids and other constituents of Salvia trichoclada. Med. Chem. Res. 4, 3831–3837 (2015). https://doi.org/10.1007/s00044-015-1424-7
Velasco, J., Holgado, F., Márquez-Ruiz, G., Ruiz-Méndez, M.V.: Concentrates of triterpenic acids obtained from crude olive pomace oils: characterization and evaluation of their potential antioxidant activity. J. Sci. Food Agric. 98, 4837–4844 (2018). https://doi.org/10.1002/jsfa.9012
Bai, X., Lai, T., Zhou, T., Li, Y., Li, X., Zhang, H.: In vitro antioxidant activities of phenols and oleanolic acid from mango peel and their cytotoxic effect on A549 cell line. Molecules 23, 1–8 (2018). https://doi.org/10.3390/molecules23061395
Acebey-Castellon, I.L., Voutquenne-Nazabadioko, L., Thi Mai, H.D., Roseau, N., Bouthagane, N., Muhammad, D., Debar, E.L.M., Gangloff, S.C., Litaudon, M., Sevenet, T., Hung, N.V., Lavaud, C.: Triterpenoid saponins from Symplocos lancifolia. J. Prod. 74, 163–168 (2011). https://doi.org/10.1021/np100502y
Horiuchi, K., Shiota, S., Hatano, T., Yoshida, T., Kuroda, T., Tsuchiya, T.: Antimicrobial activity of oleanolic acid from Salvia officinalis and related compounds on vancomycin-resistant enterococci (VRE). Biol. Pharm. Bull. 30, 1147–1149 (2007). https://doi.org/10.1248/bpb.30.1147
Chouaïb, K., Hichri, F., Nguir, A., Daami-Remadi, M., Elie, N., Touboul, D., Jannet, H.B., Hamza, M.A.: Semi-synthesis of new antimicrobial esters from the natural oleanolic and maslinic acids. Food Chem. 183, 8–17 (2015). https://doi.org/10.1016/j.foodchem.2015.03.018
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
The manuscript was written through the contributions of all authors. All authors have given approval to the final version of the manuscript. DKY, FG: supervision, conceptualization; DKY, SİB: investigation, methodology, writing-original draft, FG: writing-review and editing.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Biltekin, S.İ., Göğüş, F. & Yanık, D.K. Valorization of Olive Pomace: Extraction of Maslinic and Oleanolic by Using Closed Vessel Microwave Extraction System. Waste Biomass Valor 13, 1599–1608 (2022). https://doi.org/10.1007/s12649-021-01620-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-021-01620-w