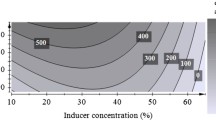
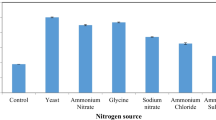
Abstract
The objective of the present work was to produce exo-polygalacturonase by solid-state fermentation of the dehydrated peels of umbu (Spondias tuberosa) using Aspergillus niger CCT 0916 and then, using a biphasic aqueous system, recover the enzyme. The peels were dehydrated at 55 °C to obtain the dried residue and then used to produce the exo-polygalacturonase using Aspergillus niger CCT 0916 and solid-state fermentation. During the 72 h fermentation, the pH, reducing sugars, and the exo-polygalacturonase activity were tested. Then the extracted enzyme was recovered with a biphasic aqueous system composed of PEG/potassium phosphate. The umbu dried residue showed a maximum peak of enzymatic activity with values of 17.30 U/g after 44 h of fermentation. The enzyme extraction using the dried umbu residue yielded a maximum PGA (Activity polygalacturonase) of 32.59 U/g. The biphasic aqueous system composed of PEG8000 and potassium phosphate salts yielded the highest recovery of 97.14%.
Graphic Abstract







Similar content being viewed by others
Data Availability
My manuscript has no associated data.
References
Gupta, N., Poddar, K., Sarkar, D., Kumari, N., Padhan, B., Sarkar, A.: Fruit waste management by pigment production and utilization of residual as bioadsorbent. J. Environ. Manag. 244, 138–143 (2019). https://doi.org/10.1016/j.jenvman.2019.05.055
Martins, Q.S.A., de Barros, H.E.A., da Cunha, S.L., Gualberto, S.A., da Silva, M.V.: Resíduos da indústria processadora de polpas de frutas: capacidade antioxidante e fatores antinutricionais. Rev. Agronegócio Meio Amb. 12(2), 591–608 (2019). https://doi.org/10.17765/2176-9168.2019v12n2p591-608
Miguel, A.C.A., Albertini, S., Begiato, G.F., Dias, J.R.P.S., Spoto, M.H.F.: Aproveitamento agroindustrial de resíduos sólidos provenientes do melão minimamente processado. LWT-Food Sci. and Technol. 28(3), 733–737 (2008). https://doi.org/10.1590/S0101-20612008000300033
Sousa, F.C., Silva, L.M.M., Lemos, D.M., Moreira, I.S., Lins, A.D.F., Castro, D.S., Rocha, A.P.T.: Secagem de residuos de Spondias sp. em camada fina. Agrotec 36(1), 197–202 (2015)
Hole, A.S., Rud, I., Grimmer, S., Sigl, S., Narvhus, J., Sahlstrøm, S.: Improved bioavailability of dietary phenolic acids in whole grain barley and oat groat following fermentation with probiotic Lactobacillus acidophilus, Lactobacillus johnsonii, and Lactobacillus reuteri. J. Agric. Food Chem. 60(25), 6369–6375 (2012). https://doi.org/10.1021/jf300410h
Mao, M., Wang, P., Shi, K., Lu, Z., Bie, X., Zhao, H., Lv, F.: Effect of solid state fermentation by Enterococcus faecalis M2 on antioxidant and nutritional properties of wheat bran. J. Cereal Sci. (2020). https://doi.org/10.1016/j.jcs.2020.102997
Soccol, C.R., da Costa, E.S.F., Letti, L.A.J., Karp, S.G., Woiciechowski, A.L., de Souza Vandenberghe, L.P.: Recent developments and innovations in solid state fermentation. Biotech. Res. Innov. 1(1), 52–71 (2017). https://doi.org/10.1016/j.biori.2017.01.002
Mandari, V., Nema, A., Devarai, S.K.: Sequential optimization and large scale production of lipase using tri-substrate mixture from Aspergillus niger MTCC 872 by solid state fermentation. Process Biochem 89, 46–54 (2020). https://doi.org/10.1016/j.procbio.2019.10.026
Prakash, G.B., Padmaja, V., Kiran, R.S.: Statistical optimization of process variables for the large-scale production of Metarhizium anisopliae conidiospores in solid-state fermentation. Bioresour. Technol. 99(6), 1530–1537 (2008). https://doi.org/10.1016/j.biortech.2007.04.031
Olukomaiya, O.O., Adiamo, O.Q., Fernando, W.C., Mereddy, R., Li, X., Sultanbawa, Y.: Effect of solid-state fermentation on proximate composition, anti-nutritional factor, microbiological and functional properties of lupin flour. Food Chem. 315, 126238 (2020). https://doi.org/10.1016/j.foodchem.2020.126238
Rayaprolu, S.J., Hettiarachchy, N.S., Chen, P., Kannan, A., Mauromostakos, A.: Peptides derived from high oleic acid soybean meals inhibit colon, liver and lung cancer cell growth. Food Res. Int. 50(1), 282–288 (2013). https://doi.org/10.1016/j.foodres.2012.10.021
Aliyah, A.N., Edelweiss, E.D., Sahlan, M., Wijanarko, A., Hermansyah, H.: Solid state fermentation using agroindustrial wastes to produce Aspergillus niger lipase as a biocatalyst immobilized by an adsorption-crosslinking method for biodiesel synthesis. Int. J. Eng. Technol. 7(8), 1393–1404 (2016). https://doi.org/10.14716/ijtech.v7i8.6988
Utami, T.S., Hariyani, I., Alamsyah, G., Hermansyah, H.: Production of dry extract extracellular lipase from Aspergillus niger by solid state fermentation method to catalyze biodiesel synthesis. Energy Procedia 136, 41–46 (2017). https://doi.org/10.1016/j.egypro.2017.10.275
Kashyap, D.R., Vohra, P.K., Chopra, S., Tewari, R.: Applications of pectinases in the commercial sector: a review. Bioresour. Technol 77(3), 215–227 (2001). https://doi.org/10.1016/S0960-8524(00)00118-8
Yu, P., Xu, C.: Production optimization, purification and characterization of a heat-tolerant acidic pectinase from Bacillus sp. ZJ1407. Int. J. Biol. Macromol. 108, 972–980 (2018). https://doi.org/10.1016/j.ijbiomac.2017.11.012
Jayani, R.S., Saxena, S., Gupta, R.: Microbial pectinolytic enzymes: a review. Process Biochem. 40(9), 2931–2944 (2005). https://doi.org/10.1016/j.procbio.2005.03.026
Lu, X., Lin, J., Wang, C., Du, X., Cai, J.: Purification and characterization of exo-polygalacturonase from Zygoascus hellenicus V25 and its potential application in fruit juice clarification. Food Sci. Biotechnol. 25(5), 1379–1385 (2016). https://doi.org/10.1007/s10068-016-0215-3
Patidar, M.K., Nighojkar, S., Kumar, A., Nighojkar, A.: Pectinolytic enzymes-solid state fermentation, assay methods and applications in fruit juice industries: a review. 3 Biotech. 8(199), 1–24 (2018). https://doi.org/10.1007/s13205-018-1220-
Amin, F., Mohsin, A., Bhatti, H.N., Bilal, M.: Production, thermodynamic characterization, and fruit juice quality improvement characteristics of an Exo-polygalacturonase from Penicillium janczewskii. BBA-proteins Proteom 1868(5), 140379 (2020). https://doi.org/10.1016/j.bbapap.2020.140379
Grilo, A.L., Barros, R.A.M., Azevedo, A.M.: Partitioning in aqueous two-phase systems: fundamentals, applications and trends. Sep. Purif. Rev. 45(1), 68–80 (2016). https://doi.org/10.1080/15422119.2014.983128
Molino, J.V.D., Marques, D.D.A.V., Júnior, A.P., Mazzola, P.G., Gatti, M.S.V.: Different types of aqueous two-phase systems for biomolecule and bioparticle extraction and purification. Biotechnol. Prog. 29(6), 1343–1353 (2013). https://doi.org/10.1002/btpr.1792
Asenjo, J.A., Andrews, B.A.: Sistemas de duas fases aquosas para separação de proteínas: uma perspectiva. J. Chromatogr. 218, 8826–8835 (2011). https://doi.org/10.1016/j.chroma.2011.06.051
Raja, S., Murty, V.R., Thivaharan, V., Rajasekar, V., Ramesh, V.: Aqueous two phase systems for the recovery of biomolecules–a review. Sci. Technol. 1(1), 7–16 (2011). https://doi.org/10.5923/j.scit.20110101.02
AOAC: Official methods of analysis of AOAC International, 20th edn. AOAC international, Rockville (2016)
Miller, G.L.: Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31(3), 426–428 (1959). https://doi.org/10.1021/ac60147a030
Ranganna, S.: Manual of analysis of fruit and vegetable products. Tata McGraw-Hill, New Delhi (2004)
Statsoft. (2007). Statistica for window—Computer programa manual (Versão 7.0). Tulsa: Statsoft Inc.
Couri, S., da Costa Terzi, S., Pinto, G.A.S., Freitas, S.P., da Costa, A.C.A.: Hydrolytic enzyme production in solid-state fermentation by Aspergillus niger 3T5B8. Process. Biochem. 36(3), 255–261 (2000). https://doi.org/10.1016/S0032-9592(00)00209-0
Souza, R.L., Oliveira, L.D.S., da Silva, F.L., Amorim, B.C.: Caracterização da poligalacturonase produzida por fermentação semi-sólida utilizando-se resíduo do maracujá como substrato. Rev. Bras. Eng. Agric. Ambient. 14(9), 987–992 (2010). https://doi.org/10.1590/S1415-43662010000900011
Castilho, L.R., Medronho, R.A., Alves, T.L.: Production and extraction of pectinases obtained by solid state fermentation of agroindustrial residues with Aspergillus niger. Bioresour. Technol. 71(1), 45–50 (2000). https://doi.org/10.1016/S0960-8524(99)00058-9
Sousa, C.A., da Silva, F.L., Conrado, L.D.S.: Lixiviação de poligalacturonases obtidas pela fermentação semissólida da casca e albedo do maracujá-amarelo. Rev. Bras. Eng. Agríc. Ambient. 16(7), 790–794 (2012). https://doi.org/10.1590/S1415-43662012000700013
Albertsson, P.A.: Partition of cell particles and macromolecules: separation and purification of biomolecules, cell organelles, membranes, and cells in aqueous polymer two-phase systems and their use in biochemical analysis and biotechnology, 3rd edn. Wiley-Interscience, New York (1986)
Zhang, Y.Y., Liu, J.H.: Purification and in situ immobilization of lipase from of a mutant of Trichosporon laibacchii using aqueous two-phase systems. J. Chromatogr. B 878(11–12), 909–912 (2010)
Bradford, M.M.: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72(1/2), 248–254 (1976). https://doi.org/10.1016/0003-2697(76)90527-3
Lima, Á.S., Alegre, R.M., Meirelles, A.J.: Partitioning of pectinolytic enzymes in polyethylene glycol/potassium phosphate aqueous two-phase systems. Carbohydr. Polym. 50(1), 63–68 (2002). https://doi.org/10.1016/S0144-8617(01)00376-9
Ascheri, D.P.R., Ascheri, J.L.R., Carvalho, C.W.P.D.: Caracterização da farinha de bagaço de jabuticaba e propriedades funcionais dos extrusados. LWT-Food Sci. Technol. 26(4), 897–905 (2006). https://doi.org/10.1590/S0101-20612006000400029
Abud, A.D.S., Narain, N.: Incorporation of fruit pulp residue flour into cookies: an alternative to combat waste. Braz. J. Food Technol. 12(1/4), 257–265 (2009). https://doi.org/10.4260/BJFT2009800900020
Franco, B.D.G.M.: Microbiologia dos alimentos, p. 182. São Paulo, Atheneu (1999)
Malvessi, E., Silveira, M.M.D.: Influence of medium composition and pH on the production of polygalacturonases by Aspergillus oryzae. Braz. Arch. Biol. Technol. 47(5), 693–702 (2004). https://doi.org/10.1590/S1516-89132004000500004
Brunauer, S., Emmett, P.H., Teller, E.: Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 60(2), 309–319 (1938). https://doi.org/10.1021/ja01269a023
Alcântara, S.R., Almeida, F.D.A., da Silva, F.L., Gomes, J.P.: Isotermas de adsorção do pedúnculo seco do caju. Rev. Bras. Eng. Agríc. Ambient. 13(1), 81–87 (2009). https://doi.org/10.1590/S1415-43662009000100012
Muniz, C.E.S., Santiago, A.M., Gusmão, T.A.S., Oliveira, H.M.L., Corando, L.S., Gusmão, R.P.: Solid-state fermentation for single-cell protein enrichment of guava and cashew by-products and inclusion on cereal bars. Biocatal Agric Biotechnol. (2020). https://doi.org/10.1016/j.bcab.2020.101576
Alexandre, H.V., Figueirêdo, R.D., Queiroz, A.D.M.: Isotermas de adsorção de umidade da pitanga em pó. Rev. Biol. Ciênc. Terra 7(1), 11–20 (2007)
Silva, H.A., Gomes, J.P., Queiroz, A.J.M.: Hygroscopic behavior and thermodynamic properties of Ziziphus joazeiro Mart Kernel Flour. J. Agric. Stud. 8(3), 50–62 (2020). https://doi.org/10.5296/jas.v8i3.15976
Leonardi, J.G., Azevedo, B.M.: Métodos de conservação de alimentos. Saúde Foco 10(1), 51–61 (2018)
Pinheiro, V.E., Desagiacomo, C.C.V., Michelin, M., Maller, A., Monteiro, L.M.O., Jorge, J.A., Polizeli, M.L.T.M.: Neosartorya glabra polygalacturonase produced from fruit peels as inducers has the potential for application in passion fruit and apple juices. Braz. J. Food Technol. 20(e2016163), 1–11 (2017). https://doi.org/10.1590/1981-6723.16316
Fontana, R.C., Salvador, S., Da Silveira, M.M.: Influence of pectin and glucose on growth and polygalacturonase production by Aspergillus niger in solid-state cultivation. J. Ind. Microbiol. Biotechnol. 32(8), 371–377 (2005). https://doi.org/10.1007/s10295-005-0004-0
Zhang, Z., Dong, J., Zhang, D., Wang, J., Qin, X., Liu, B., Xu, X., Zhang, W., Zhang, Y.: Expression and characterization of a pectin methylesterase from Aspergillus niger ZJ5 and its application in fruit processing. J. Biosci. Bioeng. 126(6), 690–696 (2018). https://doi.org/10.1016/j.jbiosc.2018.05.022
Mojsov, K.: The effects of different carbon sources on biosynthesis of pecinolytic enzymes by Aspergillus niger. Appl. Technol. Innov. 3(3), 23–29 (2010)
Maidana, S.A., Danovich, C.L., Zubreski, E.R., Martos, M.A.: Extracción de pectina a partir de albedo de limón con una Poligalacturonasa de Wickerhamomyces Anomalus. Rev. Cienc. Tecnol. 32(21), 53–63 (2019). https://doi.org/10.36995/j.recyt.2019.32.009
Gomes, J., Zeni, J., Cence, K., Toniazzo, G., Treichel, H., Valduga, E.: Evaluation of production and characterization of polygalacturonase by Aspergillus niger ATCC 9642. Food Bioprod. Process. 89(4), 281–287 (2011). https://doi.org/10.1016/j.fbp.2010.10.002
Freitas, F., Alves, V.D., Reis, M.A.M.: Advances in bacterial exopolysaccharides: from production to biotechnological applications. Trends Biol. 29(8), 388–398 (2011). https://doi.org/10.1016/j.tibtech.2011.03.008
Fan, C., Liu, Z., Hu, J., Niu, B., Huang, J.: Effects of polyethylene glycol 6000 and tripotassium phosphate on protopectinase partition in the aqueous two-phase systems using response surface methodology. Afr. J. Food Sci. 6(4), 85–90 (2012). https://doi.org/10.5897/AJFS11.209
Porfiri, M.C., Picó, G., Romanini, D., Farruggia, B.: Aspergillus oryzae alpha-amylase partition in potassium phosphate-polyethylene glycol aqueous two-phase systems. Int. J. Biol. Macromol. 49(1), 7–13 (2011). https://doi.org/10.1016/j.ijbiomac.2011.03.003
Ratanapongleka, K.: Recovery of biological products in aqueous two phase systems. Int. J. Chem. Eng. Appl. 1(2), 191 (2010)
Naganagouda, K., Mulimani, V.H.: Aqueous two-phase extraction (ATPE): an attractive and economically viable technology for downstream processing of Aspergillus oryzae α-galactosidase. Process Biochem. 43(11), 1293–1299 (2008). https://doi.org/10.1016/j.procbio.2008.07.016
Oliveira Júnior, S.D., Padilha, C.E.A., de Asevedo, E.A., de Macedo, G.R., dos Santos, E.S.: Recovery and purification of cellulolytic enzymes from Aspergillus fumigatus CCT 7873 using an aqueous two-phase micellar system. Ann. Microbiol. 70, 1–12 (2020). https://doi.org/10.1186/s13213-020-01573-w
Prodanović, J.M., Antov, M.G.: The influence of molecular weight of polyethylene glycol on separation and purification of pectinases from Penicillium cyclopium in aqueous two-phase system. Acta Period. Technol. 39, 193–199 (2008). https://doi.org/10.2298/APT0839193P
Mehrnoush, A., Sarker, M., Islam, Z., Mustafa, S., Yazid, A.M.M.: Direct purification of pectinase from mango (Mangifera Indica cv Chokanan) peel using a PEG/salt-based aqueous two phase system. Molecules 16(10), 8419–8427 (2011). https://doi.org/10.3390/molecules16108419
Silva, L.H.M.D., Loh, W.: Sistemas aquosos bifásicos: fundamentos e aplicações para partição/purificação de proteínas. Quim. Nova 29(6), 1345–1351 (2006). https://doi.org/10.1590/S0100-40422006000600033
Hemavathi, A.B., Raghavarao, K.S.M.S.: Differential partitioning of β-galactosidase and β-glucosidase using aqueous two phase extraction. Process Biochem. 46(3), 649–655 (2011). https://doi.org/10.1016/j.procbio.2010.11.008
Acknowledgements
The authors acknowledge the State University of Paraíba and the Department of Chemistry.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
ÂMS: Conceptualization, Methodology, Writing- Original draft preparation, Funding acquisition. LSCO: Funding acquisition, Reviewing and Editing PLO: Data Curation. RLJA: Statistic, Visualization. NCS: Software, Statistic. POG: Supervision Writing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Santiago, Â.M., de Sousa Conrado Oliveira, L., de Oliveira, P.L. et al. Production and Recovery of Exo-polygalacturonase from Umbu (Spondias tuberosa) Residue. Waste Biomass Valor 13, 1101–1115 (2022). https://doi.org/10.1007/s12649-021-01551-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-021-01551-6