Abstract
Purpose
Xylanases are hydrolase enzymes mainly produced by microorganisms and applied in several industrial segments. In this paper, the effects of different pretreatments of oat hulls were evaluated on the production of xylanase by Aureobasidium pullulans CCT 1261 under submerged cultivation.
Methods
Oat hulls were subjected to several pretreatments: alkaline (0.5, 1, 2, 3, and 4% of NaOH, w/v), alkaline hydrogen peroxide (7.5% of H2O2, v/v), ultrasound-assisted alkaline (1% of NaOH, w/v, 90 W, 20 kHz), and sequential alkaline and ultrasound procedures. Both untreated and pretreated oat hulls were used as substrates for xylanase production by the strain CCT 1261. Changes caused by the pretreatments were observed through the characterization of oat hulls (i.e. lignocellulosic composition and scanning electron microscopy) and then related to the enzymatic production.
Results
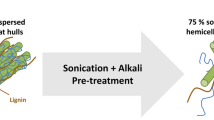
Sequential alkaline and ultrasound pretreatments on oat hulls promoted the highest xylanase production (30.2 U/mL) and productivity (0.42 U/mL.h) in comparison to the other strategies evaluated. The sequential pretreatment of oat hulls also increased xylanase production more than 11-fold when compared to the untreated substrate. The effectiveness of the sequential procedure, and consequent increase in xylanase production, were attributed to the physical modification of the biomass surface by the ultrasound waves, as well as the lignin removal and hemicellulose maintenance by the alkaline pretreatment with 1% NaOH.
Conclusion
Sequential pretreatment of oat hulls allowed the production of microbial xylanases in a more sustainable way using an agricultural by-product as substrate.
Graphic Abstract



Similar content being viewed by others
References
Bhowmick, G.D., Sarmah, A.K., Sen, R.: Lignocellulosic biorefinery as a model for sustainable development of biofuels and value added products. Bioresour. Technol. 247, 1144–1154 (2018). https://doi.org/10.1016/j.biortech.2017.09.163
Jönsson, L.J., Martín, C.: Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 199, 103–112 (2016). https://doi.org/10.1016/j.biortech.2015.10.009
Isikgor, F.H., Becer, C.R.: Lignocellulosic biomass: a sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 6, 4497–4559 (2015). https://doi.org/10.1039/C5PY00263J
Kumar, A.K., Sharma, S.: Recent updates on different methods of pretreatment of lignocellulosic feedstocks: a review. Bioresour. Bioprocess. 4, 1–19 (2017). https://doi.org/10.1186/s40643-017-0137-9
Sarkar, N., Ghosh, S.K., Bannerjee, S., Aikat, K.: Bioethanol production from agricultural wastes: An overview. Renew. Energy. 37, 19–27 (2012). https://doi.org/10.1016/j.renene.2011.06.045
Taha, M., Foda, M., Shahsavari, E., Aburto-Medina, A., Adetutu, E., Ball, A.: Commercial feasibility of lignocellulose biodegradation: possibilities and challenges. Curr. Opin. Biotechnol. 38, 190–197 (2016). https://doi.org/10.1016/j.copbio.2016.02.012
Skiba, E.A., Baibakova, O.V., Budaeva, V.V., Pavlov, I.N., Vasilishin, M.S., Makarova, E.I., Sakovich, G.V., Ovchinnikova, E.V., Banzaraktsaeva, S.P., Vernikovskaya, N.V., Chumachenko, V.A.: Pilot technology of ethanol production from oat hulls for subsequent conversion to ethylene. Chem. Eng. J. 329, 178–186 (2017). https://doi.org/10.1016/j.cej.2017.05.182
Pereira, P.H.F., Waldron, K.W., Wilson, D.R., Cunha, A.P., de Brito, E.S., Rodrigues, T.H.S., Rosa, M.F., Azeredo, H.M.C.: Wheat straw hemicelluloses added with cellulose nanocrystals and citric acid. Effect on film physical properties. Carbohydr. Polym. 164, 317–324 (2017). https://doi.org/10.1016/j.carbpol.2017.02.019
de Oliveira, J.P., Bruni, G.P., Lima, K.O., El Hallal, S.L.M., da Rosa, G.S., Dias, A.R.G., da Zavareze, E., R. : Cellulose fibers extracted from rice and oat husks and their application in hydrogel. Food Chem. 221, 153–160 (2017). https://doi.org/10.1016/j.foodchem.2016.10.048
Abaide, E.R., Ugalde, G., Di Luccio, M., Moreira, R.F.P.M., Tres, M.V., Zabot, G.L., Mazutti, M.A.: Obtaining fermentable sugars and bioproducts from rice husks by subcritical water hydrolysis in a semi-continuous mode. Bioresour. Technol. 272, 510–520 (2019). https://doi.org/10.1016/j.biortech.2018.10.075
Amorim, C., Silvério, S.C., Rodrigues, L.R.: One-step process for producing prebiotic arabino-xylooligosaccharides from brewer’s spent grain employing Trichoderma species. Food Chem. 270, 86–94 (2019). https://doi.org/10.1016/j.foodchem.2018.07.080
Varanasi, P., Singh, P., Auer, M., Adams, P.D., Simmons, B.A., Singh, S.: Survey of renewable chemicals produced from lignocellulosic biomass during ionic liquid pretreatment. Biotechnol. Biofuels. 6, 1–9 (2013). https://doi.org/10.1186/1754-6834-6-14
Pereira, G.F., de Bastiani, D., Gabardo, S., Squina, F., Ayub, M.A.Z.: Solid-state cultivation of recombinant Aspergillus nidulans to co-produce xylanase, arabinofuranosidase, and xylooligosaccharides from soybean fibre. Biocatal. Agric. Biotechnol. 15, 78–85 (2018). https://doi.org/10.1016/j.bcab.2018.05.012
Salomão, G.S.B., Agnezi, J.C., Bastos, L.P., Hencker, L.B., de Lira, T.S., Tardioli, P.W., Pinotti, L.M.: Production of cellulases by solid state fermentation using natural and pretreated sugarcane bagasse with different fungi. Biocatal. Agric. Biotechnol. 17, 1–6 (2019). https://doi.org/10.1016/j.bcab.2018.10.019
Wang, W.M., Klopfenstein, C.F.: Effect of twin-screw extrusion on the nutricional quality of wheat, barley, and oats. Cereal Chem. 70, 712–715 (1993)
Cortivo, P.R.D., Hickert, L.R., Hector, R., Ayub, M.A.Z.: Fermentation of oat and soybean hull hydrolysates into ethanol and xylitol by recombinant industrial strains of Saccharomyces cerevisiae under diverse oxygen environments. Ind. Crop. Prod. 113, 10–18 (2018). https://doi.org/10.1016/j.indcrop.2018.01.010
Tamanini, C., de Oliveira, A.S., de Felipe, M.D.G.A., Canettieri, E.V., Cândido, E.J., de Hauly, M.C.O.: Avaliação da casca de aveia para produção biotecnológica de xilitol. Acta Sci. Technol. 26, 117–125 (2004)
Demirel, F., Germec, M., Bugra, H., Irfan, C.: Optimization of dilute acid pretreatment of barley husk and oat husk and determination of their chemical composition. Cellulose 25, 6377–6393 (2018). https://doi.org/10.1007/s10570-018-2022-x
Decker, E.A., Rose, D.J., Stewart, D.: Processing of oats and the impact of processing operations on nutrition and health benefits. Br. J. Nutr. 112, 58–64 (2014). https://doi.org/10.1017/S000711451400227X
United States Departament of Agriculture (USDA): Grain: World Markets and Trade, https://www.fas.usda.gov/data/grain-world-markets-and-trade. Accessed 20 January 2021.
Dhillon, A., Gupta, J.K., Jauhari, B.M., Khanna, S.: A cellulase-poor, thermostable, alkalitolerant xylanase produced by Bacillus circulans AB 16 grown on rice straw and its application in biobleaching of eucalyptus pulp. Bioresour. Technol. 73, 273–277 (2000)
Oliveira, L.A., Porto, A.L.F., Tambourgi, E.B.: Production of xylanase and protease by Penicillium janthinellum CRC 87M–115 from different agricultural wastes. Bioresour. Technol. 97, 862–867 (2006). https://doi.org/10.1016/j.biortech.2005.04.017
Xiong, H., Weymarn, N.V., Turunen, O., Leisola, M., Pastinen, O.: Xylanase production by Trichoderma reesei Rut C-30 grown on L -arabinose-rich plant hydrolysates. Bioresour. Technol. 96, 753–759 (2005). https://doi.org/10.1016/j.biortech.2004.08.007
Uday, U.S.P., Choudhury, P., Bandyopadhyay, T.K., Bhunia, B.: Classification, mode of action and production strategy of xylanase and its application for biofuel production from water hyacinth. Int. J. Biol. Macromol. 82, 1041–1054 (2016). https://doi.org/10.1016/j.ijbiomac.2015.10.086
Shallom, D., Shoham, Y.: Microbial hemicellulases. Curr. Opin. Microbiol. 6, 219–228 (2003). https://doi.org/10.1016/S1369-5274(03)00056-0
Collins, T., Gerday, C., Feller, G.: Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiol. Rev. 29, 3–23 (2005). https://doi.org/10.1016/j.femsre.2004.06.005
Walia, A., Guleria, S., Mehta, P., Chauhan, A.: Microbial xylanases and their industrial application in pulp and paper biobleaching: a review. 3 Biotech. 7, 1–12 (2017). https://doi.org/10.1007/s13205-016-0584-6
Adhyaru, D.N., Bhatt, N.S., Modi, H.A., Divecha, J.: Cellulase-free-thermo-alkali-solvent-stable xylanase from Bacillus altitudinis DHN8: Over-production through statistical approach, purification and bio- deinking/bio-bleaching potential. Biocatal. Agric. Biotechnol. 12, 220–227 (2017). https://doi.org/10.1016/j.bcab.2017.10.010
Ghayour-Najafabadi, P., Khosravinia, H., Gheisari, A., Azarfar, A., Khanahmadi, M.: Productive performance, nutrient digestibility and intestinal morphometry in broiler chickens fed corn or wheat-based diets supplemented with bacterial- or fungal-originated xylanase. Ital. J. Anim. Sci. 17, 165–174 (2018). https://doi.org/10.1080/1828051X.2017.1328990
Abd El Aty, A.A., Saleh, S.A.A., Eid, B.M., Ibrahim, N.A., Mostafa, F.A.: Thermodynamics characterization and potential textile applications of Trichoderma longibrachiatum KT693225 xylanase. Biocatal. Agric. Biotechnol. 14, 129–137 (2018). https://doi.org/10.1016/j.bcab.2018.02.011
Boonchuay, P., Techapun, C., Leksawasdi, N., Seesuriyachan, P.: An integrated process for xylooligosaccharide and bioethanol production from corncob. Bioresour. Technol. 256, 399–407 (2018). https://doi.org/10.1016/j.biortech.2018.02.004
Shahrestani, H., Taheri-Kafrani, A., Soozanipour, A., Tavakoli, O.: Enzymatic clarification of fruit juices using xylanase immobilized on 1,3,5-triazine-functionalized silica-encapsulated magnetic nanoparticles. Biochem. Eng. J. 109, 51–58 (2016). https://doi.org/10.1016/j.bej.2015.12.013
Liu, W., Brennan, M.A., Serventi, L., Brennan, C.S.: Effect of cellulase, xylanase and α-amylase combinations on the rheological properties of Chinese steamed bread dough enriched in wheat bran. Food Chem. 234, 93–102 (2017). https://doi.org/10.1016/j.foodchem.2017.04.160
Ajijolakewu, K.A., Peng, C., Keong, C., Abdullah, W., Nadiah, W.: Characterization of novel Trichoderma hemicellulase and its use to enhance downstream processing of lignocellulosic biomass to simple fermentable sugars. Biocatal. Agric. Biotechnol. 11, 166–175 (2017). https://doi.org/10.1016/j.bcab.2017.06.005
Ding, C., Li, M., Hu, Y.: High-activity production of xylanase by Pichia stipitis: Purification, characterization, kinetic evaluation and xylooligosaccharides production. Int. J. Biol. Macromol. 117, 72–77 (2018). https://doi.org/10.1016/j.ijbiomac.2018.05.128
Menezes, B.S., Rossi, D.M., Ayub, M.A.Z.: Screening of filamentous fungi to produce xylanase and xylooligosaccharides in submerged and solid-state cultivations on rice husk, soybean hull, and spent malt as substrates. World J. Microbiol. Biotechnol. 33, 1–12 (2017). https://doi.org/10.1007/s11274-017-2226-5
Leite, P., Manuel, J., Venâncio, A., Manuel, J., Belo, I.: Ultrasounds pretreatment of olive pomace to improve xylanase and cellulase production by solid-state fermentation. Bioresour. Technol. 214, 737–746 (2016). https://doi.org/10.1016/j.biortech.2016.05.028
Bhutto, A.W., Qureshi, K., Harijan, K., Abro, R., Abbas, T., Ahmed, A., Karim, S., Yu, G.: Insight into progress in pre-treatment of lignocellulosic biomass. Energy 122, 724–745 (2017)
Sun, S., Sun, S., Cao, X., Sun, R.: The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour. Technol. 199, 49–58 (2016). https://doi.org/10.1016/j.biortech.2015.08.061
Bandikari, R., Poondla, V., Obulam, V.S.R.: Enhanced production of xylanase by solid state fermentation using Trichoderma koeningi isolate: effect of pretreated agro-residues. 3 Biotech. 4, 655–664 (2014). https://doi.org/10.1007/s13205-014-0239-4
Silva, F.L., de Campos, A.O., dos Santos, D.A., Oliveira Júnior, S.D., de Padilha, C.E.A., de Sousa Junior, F.C., de Macedo, G.R., dos Santos, E.S.: Pretreatments of Carnauba (Copernicia prunifera) straw residue for production of cellulolytic enzymes by Trichorderma reesei CCT-2768 by solid state fermentation. Renew. Energy. 116, 299–308 (2018). https://doi.org/10.1016/j.renene.2017.09.064
Sindhu, R., Binod, P., Mathew, A.K., Abraham, A., Gnansounou, E., Ummalyma, S.B., Thomas, L., Pandey, A.: Development of a novel ultrasound-assisted alkali pretreatment strategy for the production of bioethanol and xylanases from chili post harvest residue. Bioresour. Technol. 242, 146–151 (2017). https://doi.org/10.1016/j.biortech.2017.03.001
Silva, D.F., Hergesel, L.M., Campioni, T.S., Carvalho, A.F.A., Oliva-Neto, P.: Evaluation of different biological and chemical treatments in agroindustrial residues for the production of fungal glucanases and xylanases. Process Biochem. 67, 29–37 (2018). https://doi.org/10.1016/j.procbio.2018.02.008
Panwar, D., Srivastava, K.: Production, extraction and characterization of alkaline xylanase from Bacillus sp. PKD-9 with potential for poultry feed. Biocatal. Agric. Biotechnol. 3, 118–125 (2014). https://doi.org/10.1016/j.bcab.2013.09.006
Ang, S.K., Shaza, E.M., Adibah, Y.A., Suraini, A.A., Madihah, M.S.: Production of cellulases and xylanase by Aspergillus fumigatus SK1 using untreated oil palm trunk through solid state fermentation. Process Biochem. 48, 1293–1302 (2013). https://doi.org/10.1016/j.procbio.2013.06.019
dos Reis, L., Fontana, R.C., da Silva Delabona, P., da Silva Lima, D.J., Camassola, M., Pradella, J.G.C., Dillon, A.J.P.: Increased production of cellulases and xylanases by Penicillium echinulatum S1M29 in batch and fed-batch culture. Bioresour. Technol. 146, 597–603 (2013). https://doi.org/10.1016/j.biortech.2013.07.124
Otero, D.M., Cadaval, C.L., Teixeira, L.M., Rosa, C.A., Sanzo, A.V.L., Kalil, S.J.: Screening of yeasts capable of producing cellulase-free xylanase. African J. Biotechnol. 14, 1961–1969 (2015). https://doi.org/10.5897/AJB2015.14476
Adsul, M.G., Bastawde, K.B., Gokhale, D.V.: Biochemical characterization of two xylanases from yeast Pseudozyma hubeiensis producing only xylooligosaccharides. Bioresour. Technol. 100, 6488–6495 (2009). https://doi.org/10.1016/j.biortech.2009.07.064
Cavka, A., Jönsson, L.J.: Comparison of the growth of filamentous fungi and yeasts in lignocellulose-derived media. Biocatal. Agric. Biotechnol. 3, 197–204 (2014)
Rich, J.O., Leathers, T.D., Anderson, A.M., Bischoff, K.M.: Laccases from Aureobasidium pullulans. Enzyme Microb. Technol. 53, 33–37 (2013). https://doi.org/10.1016/j.enzmictec.2013.03.015
Leathers, T.D., Rich, J.O., Anderson, A.M., Manitchotpisit, P.: Lipase production by diverse phylogenetic clades of Aureobasidium pullulans. Biotechnol. Lett. 35, 1701–1706 (2013). https://doi.org/10.1007/s10529-013-1268-5
Leite, R.S.R., Alves-Prado, H.F., Cabral, H., Pagnocca, F.C., Gomes, E., Da-Silva, R.: Production and characteristics comparison of crude β-glucosidases produced by microorganisms Thermoascus aurantiacus e Aureobasidium pullulans in agricultural wastes. Enzyme Microb. Technol. 43, 391–395 (2008). https://doi.org/10.1016/j.enzmictec.2008.07.006
Lemes, A.C., Silvério, S.C., Rodrigues, S., Rodrigues, L.R.: Integrated strategy for purification of esterase from Aureobasidium pullulans. Sep. Purif. Technol. 209, 409–418 (2019). https://doi.org/10.1016/j.seppur.2018.07.062
Yegin, S., Buyukkileci, A.O., Sargin, S., Goksungur, Y.: Exploitation of Agricultural Wastes and By-Products for Production of Aureobasidium pullulans Y-2311-1 Xylanase: Screening, Bioprocess Optimization and Scale Up. Waste and Biomass Valorization. 8, 999–1010 (2017). https://doi.org/10.1007/s12649-016-9646-6
Gautério, G.V., Vieira, M.C., Gonçalves, L.G.G., Hübner, T., Sanzo, A.V.L., Kalil, S.J.: Production of xylanolitic enzymes and xylooligosaccharides by Aureobasidium pullulans CCT 1261 in submerged cultivation. Ind. Crops Prod. 125, 335–345 (2018). https://doi.org/10.1016/j.indcrop.2018.09.011
Yegin, S.: Xylanase production by Aureobasidium pullulanson globe artichoke stem: Bioprocess optimization, enzyme characterization, and application in saccharification of lignocellulosic biomass. Prep. Biochem. Biotechnol. 47, 441–449 (2016). https://doi.org/10.1080/10826068.2016.1224245
Gautério, G.V., da Silva, L.G.G., Hübner, T., da Rosa Ribeiro, T., Kalil, S.J.: Maximization of xylanase production by Aureobasidium pullulans using a by-product of rice grain milling as xylan source. Biocatal. Agric. Biotechnol. 23, 101511 (2020). https://doi.org/10.1016/j.bcab.2020.101511
AOAC: Official Methods of Analysis of International. Association of Official Analytical Chemists, Arlington (2000)
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D., Crocker, D.: Determination of Structural Carbohydrates and Lignin in Biomass. Technical Report NREL/TP-510–42618. (2008)
Goldschmid, O.: Ultraviolet spectra. In: Sarkanen, K.V., Ludwig, C.H. (eds.) Lignins: Occurrence, formation, structure and reactions, pp. 241–266. Wiley, New York (1971)
Goyal, M., Kalra, K.L., Sareen, V.K., Soni, G.: Xylanase production with xylan rich lignocellulosic wastes by a local soil isolate of Trichoderma viride. Brazilian J. Microbiol. 39, 535–541 (2008). https://doi.org/10.1590/S1517-838220080003000025
Rabelo, S.C., Andrade, R.R., Filho, R.M., Costa, A.C.: Alkaline hydrogen peroxide pretreatment, enzymatic hydrolysis and fermentation of sugarcane bagasse to ethanol. Fuel 136, 349–357 (2014). https://doi.org/10.1016/j.fuel.2014.07.033
Christov, L.P., Myburgh, J., Van Tonder, A., Prior, B.A.: Hydrolysis of extracted and fibre-bound xylan with Aureobasidium pullulans enzymes. J. Biotechnol. 55, 21–29 (1997). https://doi.org/10.1016/S0168-1656(97)00048-5
Sugumaran, K.R., Gowthami, E., Swathi, B., Elakkiya, S., Srivastava, S.N., Ravikumar, R., Gowdhaman, D., Ponnusami, V.: Production of pullulan by Aureobasidium pullulans from Asian palm kernel: A novel substrate. Carbohydr. Polym. 92, 697–703 (2013). https://doi.org/10.1016/j.carbpol.2012.09.062
Bailey, M.J., Biely, P., Poutanen, K.: Interlaboratory testing of methods for assay of xylanase activity. J. Biotechnol. 23, 257–270 (1992). https://doi.org/10.1016/0168-1656(92)90074-J
Miller, G.L.: Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31, 426–428 (1959). https://doi.org/10.1021/ac60147a030
Lowry, O.H., Rosebrough, N.J., Farr, L., Randall, R.J., Randall, R.J.: Protein measurement with the Folin phenol reagent. J. Biol. Chem. 93, 265–275 (1951)
Rodrigues, P.O., dos Santos, B.V., Costa, L., Henrique, M.A., Pasquini, D., Baffi, M.A.: Xylanase and β-glucosidase production by Aspergillus fumigatus using commercial and lignocellulosic substrates submitted to chemical pre-treatments. Ind. Crops Prod. 95, 453–459 (2017). https://doi.org/10.1016/j.indcrop.2016.10.055
Dias, L.M., Santos, B.V., Albuquerque, C.J.B., Baeta, B.E.L., Pasquini, D., Baffi, M.A.: Biomass sorghum as a novel substrate in solid-state fermentation for the production of hemicellulases and cellulases by Aspergillus niger and A. fumigatus. J. Appl. Microbiol. 124, 708–718 (2017). https://doi.org/10.1111/jam.13672
Menegol, D., Scholl, A.L., Dillon, A.J.P., Camassola, M.: Influence of different chemical pretreatments of elephant grass (Pennisetum purpureum, Schum.) used as a substrate for cellulase and xylanase production in submerged cultivation. Bioprocess Biosyst. Eng. 39, 1455–1464 (2016). https://doi.org/10.1007/s00449-016-1623-8
Wang, Z., Keshwani, D.R., Redding, A.P., Cheng, J.J.: Sodium hydroxide pretreatment and enzymatic hydrolysis of coastal Bermuda grass. Bioresour. Technol. 101, 3583–3585 (2010). https://doi.org/10.1016/j.biortech.2009.12.097
Xu, J., Cheng, J.J., Sharma-Shivappa, R.R., Burns, J.C.: Sodium hydroxide pretreatment of switchgrass for ethanol production. Energy Fuel. 24, 2113–2119 (2010). https://doi.org/10.1021/ef9014718
Kim, J.S., Lee, Y.Y., Kim, T.H.: A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 199, 42–48 (2016). https://doi.org/10.1016/j.biortech.2015.08.085
Subhedar, P.B., Gogate, P.R.: Alkaline and ultrasound assisted alkaline pretreatment for intensification of delignification process from sustainable raw-material. Ultrason. - Sonochemistry. 21, 216–225 (2014). https://doi.org/10.1016/j.ultsonch.2013.08.001
Cabrera, E., Muñoz, M.J., Martín, R., Caro, I., Curbelo, C., Díaz, A.B.: Alkaline and alkaline peroxide pretreatments at mild temperature to enhance enzymatic hydrolysis of rice hulls and straw. Bioresour. Technol. 167, 1–7 (2014). https://doi.org/10.1016/j.biortech.2014.05.103
Chen, H., Han, Y., Xu, J.: Simultaneous saccharification and fermentation of steam exploded wheat straw pretreated with alkaline peroxide. Process Biochem. 43, 1462–1466 (2008). https://doi.org/10.1016/j.procbio.2008.07.003
Hammel, K.E., Kapich, A.N., Jensen, K.A., Jr., Ryan, Z.C.: Reactive oxygen species as agents of wood decay by fungi. Enzyme Microb. Technol. 30, 445–453 (2002)
Sun, R.C., Tomkinson, J., Wang, Y.X., Xiao, B.: Physico-chemical and structural characterization of hemicelluloses from wheat straw by alkaline peroxide extraction. Polymer 41, 2647–2656 (2000). https://doi.org/10.1016/S0032-3861(99)00436-X
Soontornchaiboon, W., Kim, S.M., Pawongrat, R.: Effects of alkaline combined with ultrasonic pretreatment and enzymatic hydrolysis of agricultural wastes for high reducing sugar production. Sains Malaysiana. 45, 955–962 (2016)
Bussemaker, M.J., Zhang, D.: Effect of ultrasound on lignocellulosic biomass as a pretreatment for biorefinery and biofuel applications. Ind. Eng. Chem. Res. 52, 3563–3580 (2013). https://doi.org/10.1021/ie3022785
Acknowledgements
This study was financed in part by the “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)”–Brazil–Finance Code 001, and by the “Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)”–Brazil– Grant numbers 423285/2018-1 and 304857/2018-1. The authors are grateful to “Centro de Microscopia Eletrônica do Sul” (CEME-SUL/FURG) for SEM analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Machado, T.B., Corrêa Junior, L.C.S., de Mattos, M.V.C.d. et al. Sequential Alkaline and Ultrasound Pretreatments of Oat Hulls Improve Xylanase Production by Aureobasidium pullulans in Submerged Cultivation. Waste Biomass Valor 12, 5991–6004 (2021). https://doi.org/10.1007/s12649-021-01425-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-021-01425-x