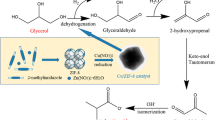
Abstract
The conversion of an oleochemical waste i.e. glycerol into lactic acid (LA) using bifunctional catalyst is receiving intensive research attention. The major concern is to achieve a suitable combination of the non-precious active metals with specific roles to achieve a fast and selective reaction. Catalyst with a Ce-to-Zr ratio of 1:2 and supported on SBA-15 at total active metal loadings between 10 and 40 wt% were prepared via a two-sequential-step post-impregnation method. The catalysts were then characterized using nitrogen adsorption–desorption and SEM analyses to elucidate their properties. They were then employed in selective glycerol oxidation reaction carried out at between 240 and 280 °C for up to 3 h to yield LA. The catalytic performance in terms of glycerol conversion and lactic acid yield was successfully correlated with the physicochemical properties of the catalysts. The highest glycerol conversion of 91.2% of glycerol was obtained using 10 wt% of CeZr/SBA-15 at a catalyst loading of 25 wt% with almost 51.4% of corresponding LA yield. It was attributed to the desired shape selectivity effect in the benzylic rearrangement (Cannizzaro reaction) of pyruvaldehyde to suppress by-product formations.
Graphic Abstract







Similar content being viewed by others
References
Abdullah, R., Saleh, S.N.M., Embong, K., Abdullah, A.Z.: Recent developments and potential advancement in the kinetics of catalytic oxidation of glycerol. Chem. Eng. Commun. (2019). https://doi.org/10.1080/00986445.2019.1641699
Wang, Y., Xiao, Y., Xiao, G.: Sustainable value-added C3 chemicals from glycerol transformations: aa mini review for heterogeneous catalytic processes. Chinese J. Chem. Eng. 27(7), 1536–1542 (2019). https://doi.org/10.1016/j.cjche.2019.03.001
Feng, Y., Yin, H., Wang, A., Gao, D., Zhu, X., Shen, L., Meng, M.: Selective oxidation of 1,2-propanediol to lactic acid catalyzed by nanosized Mg(OH)2-supported bimetallic Au–Pd catalysts. Appl. Catal. A 482, 49–60 (2014). https://doi.org/10.1016/j.apcata.2014.05.022
Yin, H., Yin, H., Wang, A., Shen, L.: Catalytic conversion of glycerol to lactic acid over graphite-supported nickel nanoparticles and reaction kinetics. J. Ind. Eng. Chem. 57, 226–235 (2018). https://doi.org/10.1016/j.jiec.2017.08.028
Feng, S., Takahashi, K., Miura, H., Shishido, T.: One-pot synthesis of lactic acid from glycerol over a Pt/L-Nb2O5 catalyst under base-free conditions. Fuel Proc. Technol. (2020). https://doi.org/10.1016/j.fuproc.2019.106202
Lakshmanan, P., Upare, P.P., Le, N.T., Hwang, Y.K., Hwang, D.W., Lee, U.H., Kim, H.R., Chang, J.S.: Facile synthesis of CeO2-supported gold nanoparticle catalysts for selective oxidation of glycerol into lactic acid. Appl. Catal. A 468, 260–268 (2013). https://doi.org/10.1016/j.apcata.2013.08.048
Maris, E.P., Davis, R.J.: Hydrogenolysis of glycerol over carbon-supported Ru and Pt catalysts. J. Catal. 249, 328–337 (2007). https://doi.org/10.1016/j.jcat.2007.05.008
Maris, E.P., Ketchie, W.C., Murayama, M., Davis, R.J.: Glycerol hydrogenolysis on carbon-supported PtRu and AuRu bimetallic catalysts. J. Catal. 251, 281–294 (2007). https://doi.org/10.1016/j.jcat.2007.08.007
Shen, Y., Zhang, S., Li, H., Ren, Y., Liu, H.: Efficient synthesis of lactic acid by aerobic oxidation of glycerol on Au–Pt/TiO2 catalysts. Chem. Eur. J. 16, 7368–7371 (2010). https://doi.org/10.1002/chem.201000740
Roy, D., Subramaniam, B., Chaudhari, R.V.: Cu-based catalysts show low temperature activity for glycerol conversion to lactic acid. ACS Catal. 1, 548–551 (2011). https://doi.org/10.1021/cs200080j
Dam, J.T., Kapteijn, F., Djanashvili, K., Hanefeld, U.: Tuning selectivity of Pt/CaCO3 in glycerol hydrogenolysis: a design of experiments approach. Catal. Commun. 13, 1–5 (2011). https://doi.org/10.1016/j.catcom.2011.06.007
Auneau, F., Michel, C., Delbecq, F., Pinel, C., Sautet, P.: Unravelling the mechanism of glycerol hydrogenolysis over rhodium catalyst through combined experimental–theoretical investigations. Chem. Eur. J. 17, 14288–14299 (2011). https://doi.org/10.1002/chem.201101318
Purushothaman, R.K.P., Haveren, J.V., Es, D.S.V., Melián-Cabrera, I., Meeldijk, J.D., Heeres, H.J.: An efficient one pot conversion of glycerol to lactic acid using bimetallic gold-platinum catalysts on a nanocrystalline CeO2 support. Appl. Catal. B 147, 92–100 (2014). https://doi.org/10.1016/j.apcatb.2013.07.068
Marques, F.L., Oliveira, A.C., Filho, J.M., Rodríguez-Castellón, E., Cavalcante, C.L., Vieira, R.S.: Synthesis of lactic acid from glycerol using a Pd/C catalyst. Fuel Process. Technol. 138, 228–235 (2015). https://doi.org/10.1016/j.fuproc.2015.05.032
Arcanjo, M.R.A., Silva, I.J., Rodríguez-Castellón, E., Infantes-Molina, A., Vieira, R.S.: Conversion of glycerol into lactic acid using Pd or Pt supported on carbon as catalyst. Catal. Today. 279, 317–326 (2017). https://doi.org/10.1016/j.cattod.2016.02.015
Kaminski, P., Ziolek, M.: Surface and catalytic properties of Ce-, Zr-, Au-, Cu-modified SBA-15. J. Catal. 312, 249–262 (2014). https://doi.org/10.1016/j.jcat.2014.02.005
Escamilla-Perea, L., Nava, R., Pawelec, B., Rosmaninho, M.G., Peza-Ledesma, C.L., Fierro, J.L.G.: SBA-15-supported gold nanoparticles decorated by CeO2: structural characteristics and CO oxidation activity. Appl. Catal. A 381, 42–53 (2010). https://doi.org/10.1016/j.apcata.2010.03.038
Saleh, S.N.M., Yusoff, M.H.M., Abdullah, A.Z.: Caesium salt of tungstophosphoric acid supported on mesoporous SBA-15 catalyst for selective esterification of lauric acid with glycerol to monolaurin. Arab. J. Sci. Eng. 43, 5771–5783 (2017). https://doi.org/10.1007/s13369-017-3009-x
Wang, F.F., Shao, S., Liu, C.L., Xu, C.L., Yang, R.Z., Dong, W.S.: Selective oxidation of glycerol over Pt supported on mesoporous carbon nitride in base-free aqueous solution. Chem. Eng. J. 264, 336–343 (2015). https://doi.org/10.1016/j.cej.2014.11.115
Gagea, B.C., Lorgouilloux, Y., Altintas, Y., Jacobs, P.A., Martens, J.A.: Bifunctional conversion of n-decane over HPW heteropoly acid incorporated into SBA-15 during synthesis. J. Catal. 265, 99–108 (2009). https://doi.org/10.1016/j.jcat.2009.04.017
Hermida, L., Abdullah, A.Z., Mohamed, A.R.: Synthesis of monoglyceride through glycerol esterification with lauric acid over propyl sulfonic acid post-synthesis functionalized SBA-15 mesoporous catalyst. Chem. Eng. J. 174, 668–676 (2011). https://doi.org/10.1016/j.cej.2011.09.072
Olutoye, M.A., Wong, S.W., Chin, L.H., Amani, H., Asif, M., Hameed, B.H.: Synthesis of fatty acid methyl esters via the transesterification of waste cooking oil by methanol with a barium-modified montmorillonite K10 catalyst. Renew. Energy. 86, 392–398 (2016). https://doi.org/10.1016/j.renene.2015.08.016
Arcanjo, M.R.A., Silva, I.J., Rodríguez-Castellón, E., Infantes-Molina, A., Vieira, R.S.: Conversion of glycerol into lactic acid using Pd or Pt supported on carbon as catalyst. Catal. Today 279, 317–326 (2017). https://doi.org/10.1016/j.cattod.2016.02.015
Hoo, P.Y., Abdullah, A.Z.: Direct synthesis of mesoporous 12-tungstophosphoric acid SBA-15 catalyst for selective esterification of glycerol and lauric acid to monolaurate. Chem. Eng. J. 250, 274–287 (2014). https://doi.org/10.1016/j.cej.2014.04.016
Moreira, A.B.F., Bruno, A.M., Souza, M.M.V.M., Manfro, R.L.: Adsorption capability of activated carbon synthesized from coconut shell. Fuel Process. Technol. 144, 170–180 (2016). https://doi.org/10.1016/j.fuproc.2015.12.025
Razali, N., Abdullah, A.Z.: Production of lactic acid from glycerol via chemical conversion using solid catalyst: a review. Appl. Catal. A 543, 234–246 (2017). https://doi.org/10.1016/j.apcata.2017.07.002
Liu, P., Derchi, M., Hensen, E.J.M.: Promotional effect of transition metal doping on the basicity and activity of calcined hydrotalcite catalysts for glycerol carbonate synthesis. Appl. Catal. B 144, 135–143 (2014). https://doi.org/10.1016/j.apcatb.2013.07.010
Kaskow, I., Decyk, P., Sobczak, I.: The effect of copper and silver on the properties of Au-ZnO catalyst and its activity in glycerol oxidation. Appl. Surf. Sci. 444, 197–207 (2018). https://doi.org/10.1016/j.apsusc.2018.02.285
Yang, G.Y., Ke, Y.H., Ren, H.F., Liu, C.L., Yang, R.Z., Dong, W.S.: The conversion of glycerol to lactic acid catalyzed by ZrO2-supported CuO catalysts. Chem. Eng. J. 283, 759–767 (2016). https://doi.org/10.1016/j.cej.2015.08.027
Kaminski, P., Ziolek, M., Bokhoven, J.A.V.: Mesoporous cerium–zirconium oxides modified with gold and copper–synthesis, characterization and performance in selective oxidation of glycerol. RSC Adv. 7, 7801–7819 (2017). https://doi.org/10.1039/c6ra27671g
Liu, S.S., Sun, K.Q., Xu, B.Q.: Specific selectivity of Au-catalyzed oxidation of glycerol and other C3-polyols in water without the presence of a base. ACS Catal. 4, 2226–2230 (2014). https://doi.org/10.1021/cs5005568
Rodrigues, A.K.O., Maia, D.L.H., Fernandes, F.A.N.: Production of lactic acid from glycerol by applying an alkaline hydrothermal process using homogeneous catalysts and high glycerol concentration. Braz. J. Chem. Eng. 32, 749–755 (2015). https://doi.org/10.1590/0104-6632.20150323s00003356
Allen, A.E., MacMillan, D.W.C.: Synergistic catalysis: a powerful synthetic strategy for new reaction development. Chem. Sci. 3, 633–658 (2012). https://doi.org/10.1039/c2sc00907b
Acknowledgements
This research was funded under the Transdisciplinary Research Grant Scheme (TRGS) (6762001) and the Fundamental Research Grant Scheme (FRGS) (6071366) that were provided by the Ministry of Education of Malaysia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saleh, S.N.M., Abdullah, A.Z. Zirconium–Cerium Oxides Supported on SBA-15 as Catalyst for Shape-Selective Synthesis of Lactic Acid from Glycerol. Waste Biomass Valor 12, 2565–2578 (2021). https://doi.org/10.1007/s12649-020-01200-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-020-01200-4