Abstract
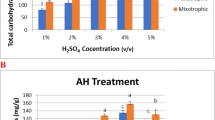
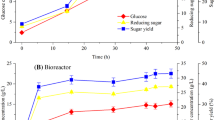
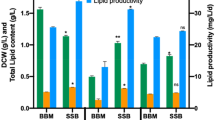
In the present study, the mixotrophic growth of the microalga Nannochloropsis sp. BR2 in sugarcane bagasse was analyzed and compared with its photoautotrophic cultivation. Nannochloropsis cultures cultivated mixotrophically in sugarcane bagasse showed significantly higher biomass productivity, fatty acid methyl ester (FAME) and protein contents of 63.28 mg L−1 d−1, 170.51 mg g−1 and 35.2% of dry weight, respectively, compared to the photoautotrophic cultivations with biomass productivity, FAME and protein contents of 51 mg L−1 d−1, 139.21 mg g−1 and 31.6% of dry weight. Whereas, total carotenoid during photoautotrophic cultivation was 5.833 mg g−1 and decreased to 4.542 mg g−1 in mixotrophic cultures. This can be explained by the additional carbon source in the form of sugars that are metabolized to the fatty acid building block acetyl-CoA, while photosynthetic pigments were less needed. Findings of this study demonstrate that acid-pretreated hydrolysate of lignocellulosic waste from sugarcane bagasse can be developed into a potential feedstock for efficient microalgal cultivation.
Graphic Abstract







Similar content being viewed by others
References
Amaro, H.M., Guedes, A.C., Malcata, F.X.: Advances and perspectives in using microalgae to produce biodiesel. Appl. Energy. 88, 3402–3410 (2011)
Manzoor, M., Tabssum, F., Javaid, H., Qazi, J.I., 2015. Lucrative future of microalgal biofuels in Pakistan: a review. Int. J. of Energy and Environ. Eng. 6, 393–403.
Moore, A.: Biofuels are dead: long live biofuels(?)-Part one. New Biotechnol. 25, 6–12 (2008)
Sukarmi, S., Hanidi, N., Yanuhar, U., Wardana, I.N.G.: Potemtial and properties of marine microalgae Nannochloropsis oculata as biomass fuel feedstock. Int. J. Energy Environ. Eng. 5(4), 279–290 (2014)
Lordan, S., Ross, R.P., Stanton, C.: Marine bioactives as functional food ingredients: potential to reduce the incidence of chronic diseases. Mar. Drugs 9, 1056–1100 (2011)
Chojnacka, K., Marquez-Rocha, F.J.: Kinetic and stoichiometric relationship of the energy and carbon metabolism in the culture of microalgae. Biotechnol. 3, 21–34 (2004)
Liang, Y., Sarkany, N., Cui, Y.: Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol. Lett. 31, 1043–1049 (2009)
Feng, F.Y., Yang, W., Jiang, G.Z., Xu, Y.N., Kuang, T.Y.: Enhancement of fatty acid production of Chlorella sp. (Chlorophyceae) by addition of glucose and sodium thiosulphate to culture medium. Process Biochem. 40, 1315–2131 (2005)
Oh, S.H., Kwon, M.C., Choi, W.Y., Seo, Y.C., Kim, G.B., Kang, D.H., Lee, S.Y., Lee, H.Y.: Long-term outdoor cultivation by perfusing spent medium for biodiesel production from Chlorella minutissima. J. Biosci. Bioeng. 110, 194–200 (2010)
Doan, Y.T.T., Obbard, J.P.: Two-stage cultivation of a Nannochloropsis mutant for biodiesel feedstock. J. Appl. Phycol. 27, 2203–2208 (2015)
Rodolfi, L., Chini Zittelli, G., Bassi, N., Padovani, G., Biondi, N., Bonini, G., et al.: Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bioeng. 102, 100–112 (2009)
Tang, G., Suter, P.M.: Vitamin A, nutrition, and health values of algae: spirulina, chlorella, and dunaliella. J. Pharm. Nutr. Sci. 1, 111–118 (2011)
Kim, M.K., Jeune, K.-H.: Use of FT-IR to identify enhanced biomass production and biochemical pool shifts in the marine microalgae, Chlorella ovalis, cultured in media composed of different ratios of deep seawater and fermented animal wastewater. J. Microbiol. Biotechnol. 19, 1206–1212 (2009)
Craggs, R.J., McAuley, P.J., Smith, V.J.: Wastewater nutrient removal by marine microalgae grown on a corrugated raceway. Water Res. 31, 1701–1707 (1997)
Ma, X.N., Chen, T.P., Yang, B., Liu, J., Chen, F.: Lipid production from Nannochloropsis. Mar. Drugs 14, 61 (2016)
Chua, E.T., Schenk, P.M.: A biorefinery for Nannochloropsis: induction, harvesting and extraction of EPA-rich oil and high-value protein. Biores. Technol. 244, 1416–1424 (2017)
deVrije, T., Claassen, P.A.M.: Dark hydrogen fermentations. In: Reith, J.H., Wijffels, R.H., Barten, H. (eds.) Bio-methane & Bio-hydrogen, pp. 103–123. Smiet Offset, The Hague (2003)
Villareal, M.L.M., Prata, A.M.R., Felipe, M.G.A., Almeida, E., Silva, E.: Detoxification procedure of eucalyptus hemicelluloses hydrolysate for xylitol production of Candida gulliermondii. Enzyme Microb. Technol. 40, 17–24 (2006)
Klement, T., Milker, S., Jäger, G., Grande, P.M., de-Maria, D., Büchs, J.: Biomass pretreatment affects Ustilago maydis in producing itaconic acid. Microb. Cell Factories. 11, 43 (2012)
Rattanapoltee, P., Kaewkannetra, P.: Utilization of agricultural residues of pineapple peels and sugarcane bagasse as cost-saving raw materials in Scenedesmus acutus for lipid accumulation and biodiesel production. Appl. Biochem. Biotechnol. 173, 1495–1510 (2014)
Huang, C., Chen, X.F., Xiong, L., Chen, X.D., Ma, L.L., Chen, Y.: Single cell oil production from low-cost substrates: the possibility and potential of its industrialization. Biotechnol. Adv. 31, 129–139 (2013)
Taherzadeh, M.J., Karimi, K.: Pretreatment of lognocellulosic wastes to improve ethanol and biogas production: a review. Int. J. Mol. Sci. 9, 1621–1651 (2008)
Sun, J.X., Sun, X.F., Zhao, H., Sun, R.C.: Isolation and characterization of cellulose from sugarcane bagasse. Polym. Degrad. Stab. 84, 331–339 (2004)
Manzoor, M., Jabeen, F., Skye, R.T.-H., Altaf, J., Younis, T., Schenk, P.M., Qazi, J.I.: Sugarcane bagasse as a novel low/no cost organic carbon source for growth of Chlorella sp. BR2. Biofuels. 10, 1–7 (2019)
Manzoor, M., Ahmad, Q.-A., Aslam, A., Jabeen, F., Rasul, A., Schenk, P.M., Qazi, J.I.: Mixotrophic cultivation of Scenedesmus dimorphus in sugarcane bagasse hydrolysate. Environ. Prog. Sustain. Energy. 39, e13334 (2020)
Lim, D.K.Y., Garg, S., Timmins, M., Zhang, E.S.B., Thomas-Hall, S.R., Schuhmann, H., et al.: Isolation and evaluation of oil producing microalgae from subtropical coastal and brackish waters. PLoS ONE 7, e40751 (2012)
Guilard, R.R.L., Ryther, J.H.: Studies of marine planktonic diatoms. I. cyclotella nana hustedt and Detonula confervacea. Cleve. Can. J. Microbiol. 8, 229–239 (1962)
Ho, S., Chen, H.C.Y., Chang, J.S.: Effect of light intensity and nitrogen starvation on CO2 fixation and lipid/carbohydrate production of an indigenous microalga Scenedesmus obliquus CNW-N. Bioresour. Technol. 113, 224–252 (2012)
Segal, L., Creely, J., Martin, A., Conard, C.: An empirical method for estimating the degree of crystallinity of native cellulose using the X- ray diffractometer. Text Res. J. 29, 786–794 (1959)
Rodrigues, F.G., Assuncăõ, R.M.N., Vieira, J.G., Meireles, C.S., Cerqueira, D.A., Barud, H.S., Ribeiro, S.J.L., Messaddeq, Y.: Characterization of methylcellulose produced from sugarcane bagasse cellulose: Crystallinity and thermal properties. Polym. Degrad. Stab. 92, 205–210 (2007)
Ahmed, F., Fanning, K., Netzel, M., Turner, W., Li, Y., Schenk, P.M.: Profiling of carotenoids and antioxidant capacity of microalgae from subtropical coastal and brackish waters. Food Chem. 165, 300–306 (2014)
Jeffery, S., Humphrey, G.: New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural populations. Biochem. Physiol. Pflanz. 167, 191–194 (1975)
Lopez, C.V.G., Garcia, C.C., Fernandes, F.G.A., Bustos, C.S., Chisti, Y., Sevila, J.M.F.: Protein measurements of microalgal and cyanobacterial biomass. Bioresour. Technol. 101, 7587–7591 (2010)
Fogg, G.E.: Some comments on picoplankton and its importance in the pelagic ecosystem. Aquat. Microb. Ecol. 9, 33–39 (1995)
Volkman, J.K., Brown, M.R., Dunstan, G.A., Jeffrey, S.: The biochemical composition of marine microalgae from the class eustigmatophyceae. J. Phycol. 29, 69–78 (1993)
Lubián, L.M., Montero, O., Moreno-Garrido, I., Huertas, I.E., Sobrino, C., González-del Valle, M., et al.: Nannochloropsis (eustigmatophyceae) as source of commercially valuable pigments. J. Appl. Phycol. 12, 249–255 (2000)
Lee, M.-Y., Min, B.-S., Chang, C.-S., Jin, E.: Isolation and characterization of a xanthophyll aberrant mutant of the green alga Nannochloropsis oculata. Mar. Biotechnol. 8, 238–245 (2006)
Osinga, R., Kleijn, R., Groenendijk, E., Niesink, P., Tramper, J., Wijffels, R.H.: Development of in vivo sponge cultures: particle feeding by the tropical sponge Pseudosuberites aff. andrewsi. Mar. Biotechnol. 3, 544–554 (2001)
Ferreira, M., Coutinho, P., Seixas, P., Fábregas, J., Otero, A.: Enriching rotifers with ‘‘premium’’ microalgae Nannochloropsis gaditana. Mar. Biotechnol. 11, 585–595 (2009)
Griffiths, M.J., Harrison, S.T.L.: Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J. Appl. Phycol. 21, 493–507 (2009)
Heredia-Arroyo, T., Wei, W., Hu, B.: Oil accumulation via heterotrophic/mixotrophic Chlorella protothecoides. Appl. Biochem. Biotechnol. 162, 1978–1995 (2010)
Hu, H., Gao, K.: Optimization of growth and fatty acid composition of unicellular marine picoplankton, Nannochloropsis sp., with enriched carbon sources. Biotechnol. Lett. 25(5), 421–425 (2003)
Xu, N., Zhang, W., Ren, S., Liu, F., Zhao, C., Liao, H., Xu, Z., et al.: Hemicelluloses negatively affect lignocellulose crystallinity for high biomass digestibility under NaOH and H2SO4 pretreatments in Miscanthus. Biotechol. Biofuels 5, 58 (2012)
Pardo, M.F., Mwndoza, J.G.S., Galán, J.E.L.: Influence of pretreatments on crystallinity and enzymatic hydrolysis in sugarcane residues. Braz. J. Chem. Eng. 36(1), 131–141 (2019)
Rezendze, C.A., Delima, M.A., Maziero, P., Deazevedo, E.R., Garcia, W., Polikarpov, I.: Chemical and morphological characterization of sugarcane bagasse submitted to a delignification process for enhanced enzymatic digestibility. Biotechnol. Biofuels. 11, 4–54 (2011)
Chandel, A.K., Antunes, F.A.F., Anjos, V., Bell, M.J.V., Rodrigues, L.N., Polikarpov, I., Azevedo, E.R., Bernardinelli, O.D., Rosa, C.A., Pagnocca, F.C., Silva, S.S.: Multi-scale structural and chemical analysis of sugarcane bagasse in the process of sequential acid–base pretreatment and ethanol production by Scheffersomyces shehatae and Saccharomyces cerevisiae. Biotechnol. Biofuels 7, 63 (2014)
Sindhu, R., Binod, P., Satyanagalakshmi, K., Janu, K.U., Sajna, K.V., Kurien, N., Sukumaran, R.K., Pandey, A.: Formic acid as a potential pretreatment agent for the conversion of sugarcane bagasse to bioethanol. Appl. Biochem. Biotechnol. 162, 2313–2323 (2010)
Xu, F., Cong, W., Cai, Z.-L., Ouyang, F.: Effects of organic carbon sources on cell growth and eicosapentaenoic acid content of Nannochloropsis sp. J. Appl. Phycol. 16, 499–503 (2004)
Gao, C.F., Zhai, Y., Ding, Y., Wu, Q.Y.: Application of sweet sorghum for biodiesel production by heterotrophic microalga Chlorella protothecoides. Appl. Energy 87, 756–761 (2010)
Cerón-García, M.C., Macías-Sánchez, M.D., Sánchez-Mirón, A., García-Camacho, F., Molina-Grima, E.: A process for biodiesel production involving the heterotrophic fermentation of Chlorella protothecoides with glycerol as the carbon source. Appl. Energy. 103, 341–349 (2013)
Wang, W.R., Zhou, W.W., Liu, J., Li, Y.H., Zhang, Y.K.: Biodiesel production from hydrolysate of Cyperus esculentus waste by Ch1orella vulgaris. Bioresour. Technol. 136, 24–29 (2013)
Silva, H.R., Prete, C.E.C., Zambrano, F., de-Mello, V.H., Tischer, C.A., Andrade, A.S.: Combining glucose and sodium acetate improves the growth of Neochloris oleabundans under mixotrophic conditions. AMB Express 6, 10 (2016)
Cheirisilp, B., Torpee, S.: Enhanced growth and lipid production of microalgae under mixotrophic culture condition: effect of light intensity, glucose concentration and fed-batch cultivation. Bioresour. Technol. 110, 510–516 (2012)
Kong, W., Song, H., Cao, Y., Yang, H., Hua, S., Xia, C.: The characteristics of biomass production, lipid accumulation and chlorophyll biosynthesis of Chlorella vulgaris under mixotrophic cultivation. Afr. J. Biotech. 10, 11620–11630 (2011)
Lee, Y.-K., Ding, S.-Y., Hoe, C.-H., Low, C.-S.: Mixotrophic growth of Chlorella sorokiniana in outdoor enclosed photobioreactor. J. Appl. Phycol. 8, 163–169 (1996)
Pagnanelli, F., Altimari, P., Trabucco, F., Toro, L.: Mixotrophic growth of Chlorella vulgaris and Nannochloropsis oculata: interaction between glucose and nitrate. J. Chem. Technol. Biotechnol. 89, 652–661 (2014)
Salati, S., Dimorzano, G., Menin, B., Veronesi, D., Scagila, B., Abruscato, P., Mariani, P., Adani, F.: Mixotrophic cultivation of Chlorella for local protein production using agro-food by-products. Bioresour. Technol. 230, 82–89 (2017)
Sforza, E., Cipriani, R., Morosinotto, T., Bertucco, A., Giacometti, G.M.: Excess CO2 supply inhibits mixotrophic growth of Chlorella protothecoides and Nannochloropsis salina. Bioresour. Technol. 104, 523–529 (2012)
Yu, H., Jia, S., Dai, Y.: Growth characteristics of the cyanobacterium Nostoc flagelliforme in photoautotrophic, mixotrophic and heterotrophic cultivation. J. Appl. Phycol. 21, 127–133 (2008)
Mu, J., Li, S., Chen, D., Xu, H., Han, F., Feng, B., Li, Y.: Enhanced biomass and oil production from sugarcane bagasse hydrolysate (SBH) by heterotrophic oleaginous microalga Chlorella protothecoides. Bioresour. Technol. 185, 99–105 (2015)
Wan, M., Liu, P., Xia, J., Rosenberg, J.N., Oyler, G.A., Betenbaugh, M.J., Qiu, G.D.: The effect of mixotrophy on microalgal growth, lipid content, and expression levels of three pathway genes in Chlorella sorokiniana. Appl. Microbiol. Biotechnol. 91, 835–844 (2011)
Zhao, G., Yu, J., Jiang, F., Zhang, X., Tan, T.: The effect of different trophic modes on lipid accumulation of Scenedesmus quadricauda. Bioresour. Technol. 114, 466–471 (2012)
Arora, N., Patel, A., Pruthi, P.A., Pruthi, V.: Boosting TAG Accumulation with Improved Biodiesel Production from Novel Oleaginous Microalgae Scenedesmus sp. IITRIND2 Utilizing Waste Sugarcane Bagasse Aqueous Extract (SBAE). Appl. Biochem. Biotechnol. 180, 109–121 (2016)
Fang, X., Wei, C., Zhao-Ling, C., Fan, O.: Effects of organic carbon sources on cell growth and eicosapentaenoic acid content of Nannochloropsis sp. J. Appl. Phycol. 16, 499–503 (2004)
Suen, Y., Hubbard, J.S., Holzer, G., Tornabene, T.G.: Total lipid production of the green alga Nannochloropsis sp. QII under different nitrogen regimes. J. Phycol. 23, 289–296 (1987)
Sevilla, J.M.F., García, M.C.C., Mirón, A.S., Belarbi, H., Garcia, C.F., Molina, E.G.: Pilot-plant-scale outdoor mixotrophic cultures of Phaeodactylum tricornutum using glycerol in vertical bubble column and airlift photobioreactors: studies in fed-batch mode. Biotechnol. Progress. 20, 728–736 (2004)
Wood, B.J.B., Grimson, P.H.K., German, G.B., Turner, M.: Photoheterotrophy in the production of phytoplankton organisms. J. Biotechnol. 70, 175–183 (1999)
Saraf, S., Thomas, B.: Influence of feedstock and process chemistry on biodiesel quality. Process Saf. Environ. Prot. 85, 360–364 (2007)
Lin, T.S., Wu, J.Y.: Effect of carbon sources on growth and lipid accumulation of newly isolated microalgae cultured under mixotrophic condition. Bioresour. Technol. 184, 100–107 (2015)
Miazek, K., Remacle, C., Richel, A., Goffin, D.: Beech wood Fagus sylvatica dilute-acid hydrolysate as a feedstock to support Chlorella sorokiniana biomass, fatty acid and pigment production. Bioresour. Technol. 230, 122–131 (2017)
Nguyen, H.C., Liang, S.-H., Chen, S.-S., Su, C.-H., Lin, J.-H., Chien, C.-C.: Enzymatic production of biodiesel from insect fat using methyl acetate as an acyl acceptor: optimization by using response surface methodology. Energy Convers. Manage. 158, 168–175 (2018)
Knothe, G.: A technical evaluation of biodiesel from vegetable oils vs. algae. Will algae derived biodiesel perform? Green Chem. 13, 3048–3065 (2011)
Cho, K., Lee, C.H., Ko, K., Lee, Y.-L., Kim, K.-N., Kim, M.-K., Chung, Y.-H., Kim, I.D., Yeo, K., Oda, T.: Use of phenol-induced oxidative stress acclimation to stimulate cell growth and biodiesel production by the oceanic microalga Dunaliella salina. Algal Res. 17, 61–66 (2016)
Yang, C., Hua, Q., Shimizu, K.: Energetics and carbon metabolism during growth of microalgal cells under photoautotrophic, mixotrophic and cyclic light-autotrophic/dark-heterotrophic conditions. Biochem. Eng. J. 6, 87–102 (2000)
Ip, P.F., Wong, K.H., Chen, F.: Enhanced production of astaxanthin by the green microalga Chlorella zofingiensis in mixotrophic culture. Process. Biochem. 39, 1761–1766 (2004)
Yan, R., Zhu, D., Zhang, Z., Zeng, Q., Chu, J.: Carbon metabolism and energy conversion of Synechococcus sp. PCC7942 under mixotrophic conditions: comparison with photoautotrophic conditions. J. Appl. Phycol. 24, 657–668 (2012)
Liu, X., Duan, S., Li, A., Xu, N., Cai, Z., Hu, Z.: Effects of organic carbon sources on growth, photosynthesis, and respiration of Phaeodactylum tricornutum. J. Appl. Phycol. 21, 239–246 (2009)
Abreu, A.P., Fernandes, B., Vicente, A.A., Teixeira, J., Dragone, G.: Mixotrophic cultivation of Chlorella vulgaris using industrial dairy waste as organic carbon source. Bioresour. Technol. 118, 61–66 (2012)
Wang, J., Yang, H., Wang, F.: Mixotrophic cultivation of microalgae for biodiesel production: status and prospects. Appl. Biochem. Biotechnol. 172, 3307–3329 (2014)
Laliberté, G., Noüie, J.: Auto-, hetero- and mixotrophic growth of Chlamydomonas humicola (Chloroimyckak) on acetate. J. Phycol. 29, 612–620 (1993)
Hassall, K.A.: Xylose as a specific inhibitor of photosynthesis. Nature 181, 1273–1274 (1958)
Leite, G.B., Paranjape, K., Abdelaziz, A.E.M., Hallenbeck, P.C.: Utilization of biodiesel derived glycerol or xylose for increased growth and lipid production by indigenous microalgae. Bioresour. Technol. 184, 123–130 (2015)
Leite, G.B., Paranjape, K., Hallenbeck, P.C.: Breakfast of champions: fast lipid accumulation by cultures of Chlorella and Scenedesmus induced by xylose. Algal Res. 16, 338–348 (2016)
Griffiths, D.J., Thresler, C.L., Street, H.E.: The heterotrophic nutrition of Chlorella vulgaris. Ann. Bot. 2, 1–11 (1960)
Perez-Garcia, O., Escalante, F.M.E., de-Bashan, L.E., Bashan, Y.: Heterotrophic cultures of microalgae: metabolism and potential products. Water Res. 45, 11–36 (2011)
Cardona, C.A., Quintero, J.A., Paz, I.C.: Production of bioethanol from sugarcane bagasse: status and perspectives. Bioresour. Technol. 101, 4754–4766 (2010)
Gabov, K., Hemming, J., Fardim, P.: Sugarcane bagasse valorization by fractionation using a water-based hydrotropic process. Ind. Crops Prod. 108, 495–504 (2017)
Acknowledgements
We acknowledge the Higher Education Commission of Pakistan to provide financial assistance for the first author to conduct the present study.
Author information
Authors and Affiliations
Contributions
MM, did all experimentation and contributed to manuscript writing. FJ, QAH, TY, helped in statistical analysis and acquisition of data. EE, helped in analyzing results and with experiments. PMS, contributed to study design, critical discussions and revision of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Manzoor, M., Jabeen, F., Ahmad, QuA. et al. Sugarcane Bagasse Hydrolysate as Organic Carbon Substrate for Mixotrophic Cultivation of Nannochloropsis sp. BR2. Waste Biomass Valor 12, 2321–2331 (2021). https://doi.org/10.1007/s12649-020-01185-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-020-01185-0