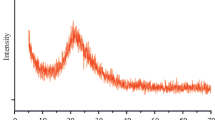
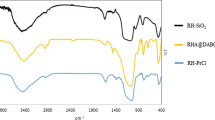
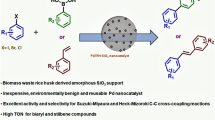
Abstract
Molybdenum and lanthanum oxide modified silica-based catalysts were prepared from the agricultural waste rice husk. These synthesized catalysts were characterized by various spectroscopic and non-spectroscopic techniques. The catalytic performance was investigated by transesterification reaction between diethyl malonate and benzyl alcohol in the liquid phase using modified silica as a heterogeneous catalyst. Molybdenum modified silica-based catalyst showed the highest conversion efficiency of 95.6% and selectivity of 96.8% for dibenzyl malonate. The reaction conditions were optimized to give maximum efficiency with the highest selectivity in a solvent-free green method.
Graphic Abstract













Similar content being viewed by others
References
Shahla, S., Cheng, N.G., Yusoff, R.: An overview on transesterification of natural oils and fats. Biotechnol. Bioprocess Eng. 1(15), 891–904 (2010). https://doi.org/10.1007/s12257-009-3157-2
Bittner, S., Felix, S., Authority, D., Sheva, B.: A convenient method of transesterification under neutral conditions. Tetrahedron Lett. 44, 3871–3874 (1975). https://doi.org/10.1016/S0040-4039(00)91300-5
Raghavendra, P.S., Shamshuddin, M.S.Z., Thimmaraju, N.: Vapor phase transesterification of dimethyl malonate with phenol over cordierite honeycomb coated with zirconia and its modified forms. Int. J. Chem. Mol. Eng. 9, 1383–1386 (2015). https://doi.org/10.5281/zenodo.1110363
Zhang, C., Li, S., Bao, S.: Sustainable synthesis of ZSM-5 zeolite from rice husk ash without addition of solvents. Waste Biomass Valoriz. (2018). https://doi.org/10.1007/s12649-018-0356-0
Andreola, F., Lancellotti, I., Manfredini, T., Bondioli, F., Barbieri, L.: Rice husk ash (RHA) recycling in brick manufactureeffects on physical and microstructural properties. Waste Biomass Valoriz. 9, 2529–2539 (2018). https://doi.org/10.1007/s12649-018-0343-5
Demis, S., Tapali, J.G., Papadakis, V.G.: Plant design and economics of rice husk ash exploitation as a pozzolanic material. Waste Biomass Valoriz. 6, 843–853 (2015). https://doi.org/10.1007/s12649-015-9412-1
Rosa, D.S., Vargas, B.P., Silveira, M.V., Rosa, C.H., Martins, M.L., Rosa, G.R.: On the use of calcined agro-industrial waste as palladium supports in the production of eco-friendly catalysts : rice husks and banana peels tested in the Suzuki–Miyaura reaction. Waste Biomass Valoriz. (2018). https://doi.org/10.1007/s12649-018-0252-7
Radhika, T., Sugunan, S.: Structural and catalytic investigation of vanadia supported on ceria promoted with high surface area rice husk silica. J. Mol. Catal. A Chem. 250, 169–176 (2006). https://doi.org/10.1016/j.molcata.2006.01.048
Thimmaraju, N., Shamshuddin, S.Z.M., Pratap, S.R.: Transesterification of diethyl malonate with benzyl alcohol catalyzed by modified zirconia: kinetic study. J. Mol. Catal. A Chem. 391, 55–65 (2014). https://doi.org/10.1016/j.molcata.2014.03.025
Saravanamurugan, S., Han, D., Koo, J., Park, S.: Transesterification reactions over morphology controlled amino-functionalized SBA-15 catalysts. Catal. Commun. 9, 158–163 (2008). https://doi.org/10.1016/j.catcom.2007.06.002
Shah, P., Ramaswamy, A.V., Lazar, K., Ramaswamy, V.: Synthesis and characterization of tin oxide-modified mesoporous SBA-15 molecular sieves and catalytic activity in trans-esterification reaction. Appl. Catal. A Gen. 273, 239–248 (2004). https://doi.org/10.1016/j.apcata.2004.06.039
Yadav, G.D., Kadam, A.A.: Selective engineering using Mg–Al calcined hydrotalcite and microwave irradiation in mono-transesterification of diethyl malonate with cyclohexanol. Chem. Eng. J. 230, 547–557 (2013). https://doi.org/10.1016/j.cej.2013.06.075
Vijayasankar, A.V., Nagaraju, N.: Preparation and characterisation of amorphous mesoporous aluminophosphate and metal aluminophosphate as an efficient heterogeneous catalyst for transesterification reaction. Comptes Rendus Chim. 14, 1109–1116 (2011). https://doi.org/10.1016/j.crci.2011.09.013
Romero, E., Soto, R., Durán, P., Herguido, J., Peña, J.A.: Molybdenum addition to modified iron oxides for improving hydrogen separation in fixed bed by redox processes. Int. J. Hydrogen Energy. 37, 6978–6984 (2012). https://doi.org/10.1016/j.ijhydene.2011.11.066
González, J., Wang, J.A., Chen, L., Manríquez, M., Salmones, J., Limas, R., Arellano, U.: Quantitative determination of oxygen defects, surface Lewis acidity, and catalytic properties of mesoporous MoO3/SBA-15 catalysts. J. Solid State Chem. 263, 100–114 (2018). https://doi.org/10.1016/j.jssc.2018.04.005
Al-yassir, N., Mao, R.L.Van: Physico-chemical properties of mixed molybdenum and cerium oxides supported on silica—alumina and their use as catalysts in the thermal-catalytic cracking (TCC) of n-hexane. Appl. Catal. A Gen. 305(305), 130–139 (2006). https://doi.org/10.1016/j.apcata.2006.02.054
Sugunan, S., Sherly, K.B.: Basicity and electron donor properties of lanthanum oxide and its mixed oxides with alumina. Indian J. Chem. 32A, 689–692 (1993)
Katta, L., Sudarsanam, P., Mallesham, B., Reddy, B.M.: Preparation of silica supported ceria-lanthana solid solutions useful for synthesis of 4-methylpent-1-ene and dehydroacetic acid. Catal. Sci. Technol. 2, 995–1004 (2012). https://doi.org/10.1039/c2cy00551d
Mekhemer, G.A.H.: Surface structure and acid–base properties of lanthanum oxide dispersed on silica and alumina catalysts. Phys. Chem. Chem. Phys. 4, 5400–5405 (2002). https://doi.org/10.1039/b204056p
Statman, D.J., Gleaves, J.T., McNamara, D., Mills, P.L., Fornasari, G., Ross, J.R.H.: TAP reactor investigation of methane coupling over samarium oxide catalysts. Appl. Catal. 77, 45–53 (1991). https://doi.org/10.1016/0166-9834(91)80022-O
Bindig, R., Liu, D., Enke, D., Wohlrab, S., Seeburg, D., Kreft, S., Hartmann, I., Schneider, D.: Rice husk derived porous silica as support for Pd and CeO2 for low temperature catalytic methane combustion. Catalysts. 9, 26 (2019). https://doi.org/10.3390/catal9010026
Vilanculo, C.B., de Andrade Leles, L.C., da Silva, M.J.: H4SiW12O40-catalyzed levulinic acid esterification at room temperature for production of fuel bioadditives. Waste Biomass Valoriz. (2018). https://doi.org/10.1007/s12649-018-00549-x
Dintzer, T., Petit, C., Petit, P., Ersen, O., Luo, J., Chu, W.: Anchoring and promotion effects of metal oxides on silica supported catalytic gold nanoparticles. J. Colloid Interface Sci. 482, 135–141 (2016). https://doi.org/10.1016/j.jcis.2016.08.001
Tomke, P.D., Zhao, X., Chiplunkar, P.P., Xu, B., Wang, H., Silva, C., Rathod, V.K., Cavaco-Paulo, A.: Lipase-ultrasound assisted synthesis of polyesters. Ultrason. Sonochem. 38, 496–502 (2017). https://doi.org/10.1016/j.ultsonch.2017.03.051
Reed, R.H., Chilvers, K.F., James, A.L., Morris, K.A., Oliver, M., Perry, J.D., Gould, F.K.: Evaluation of novel fluorogenic substrates for the detection of glycosidases in Escherichia coli and enterococci. J. Appl. Microbiol. 101, 977–985 (2006). https://doi.org/10.1111/j.1365-2672.2006.03018.x
Brandau, S., Landa, A., Franzén, J., Marigo, M., Jørgensen, K.A.: Organocatalytic conjugate addition of malonates to α, β-unsaturated aldehydes: asymmetric formal synthesis of (-)-paroxetine, chiral lactams, and lactones. Angew. Chem. Int. Ed. 45, 4305–4309 (2006). https://doi.org/10.1002/anie.200601025
Hemalatha, P., Bhagiyalakshmi, M., Ganesh, M., Palanichamy, M., Murugesan, V., Jang, H.T.: Role of ceria in CO2 adsorption on NaZSM-5 synthesized using rice husk ash. J. Ind. Eng. Chem. 18, 260–265 (2012). https://doi.org/10.1016/j.jiec.2011.11.046
Adam, F., Thankappan, R.: Oxidation of benzene over bimetallic Cu–Ce incorporated rice husk silica catalysts. Chem. Eng. J. 160, 249–258 (2010). https://doi.org/10.1016/j.cej.2010.02.055
Renu, P., Radhika, T., Suganan, S.: Characterization and catalytic activity of Vanadia supported on rice husk silica promoted samaria. Catal. Commun. 9, 584–589 (2008). https://doi.org/10.1016/j.catcom.2007.02.024
Reddy, B.M., Chowdhury, B., Smirniotis, P.G.: An XPS study of La2O3 and In2O3 influence on the physicochemical properties of MoO3/TiO2 catalysts. Appl. Catal. A Gen. 219, 53–60 (2001). https://doi.org/10.1016/S0926-860X(01)00658-5
Elassal, Z., Groula, L., Nohair, K., Sahibed-dine, A., Brahmi, R., Loghmarti, M., Mzerd, A., Bensitel, M.: Synthesis and FT-IR study of the acido-basic properties of the V2O5 catalysts supported on zirconia. Arab. J. Chem. 4, 313–319 (2011). https://doi.org/10.1016/j.arabjc.2010.06.052
Shamshuddin, S.Z.M., Nagaraju, N.: Vapour phase synthesis of salol over solid acids via transesterification. J. Chem. Sci. 122, 193–201 (2010). https://doi.org/10.1007/s12039-010-0022-y
Thimmaraju, N., Pratap, S.R., Senthilkumar, M., Shamshuddin, S.Z.M.: Honeycomb monolith coated with Mo(VI)/ZrO2 as a versatile catalyst system for liquid phase transesterificaiton. J. Korean Chem. Soc. 56, 563–570 (2012). https://doi.org/10.5012/jkcs.2012.56.5.563
Saravanamurugan, S., Sujandi, S., Han, D.S., Koo, J.B., Park, S.E.: Transesterification reactions over morphology controlled amino-functionalized SBA-15 catalysts. Catal. Commun. 9, 158–163 (2008). https://doi.org/10.1016/j.catcom.2007.06.002
Ajaikumar, S., Backiaraj, M., Mikkola, J.P., Pandurangan, A.: Transesterification of diethyl malonate with n-butanol over HPWA/MCM-41 molecular sieves. J. Porous Mater. 20, 951–959 (2013). https://doi.org/10.1007/s10934-013-9672-8
Minchitha, K.U., Hareesh, H.N., Venkatesh, K., Shanty, M., Nagaraju, N., Kathyayini, N.: Design of sulphate modified solid acid catalysts for transesterification of diethyl malonate with benzyl alcohol. J. Nanosci. Nanotechnol. 18, 202–214 (2018). https://doi.org/10.1166/jnn.2018.14567
Biradar, A.V., Umbarkar, S.B., Dongare, M.K.: Transesterification of diethyl oxalate with phenol using MoO3/SiO2 catalyst. Appl. Catal. A Gen. 285, 190–195 (2005). https://doi.org/10.1016/j.apcata.2005.02.028
Ma, X., Gong, J., Yang, X., Wang, S.: A comparative study of supported MoO3 catalysts prepared by the new “slurry” impregnation method and by the conventional method: their activity in transesterification of dimethyl oxalate and phenol. Appl. Catal. A Gen. 280, 215–223 (2005). https://doi.org/10.1016/j.apcata.2004.11.001
Kotbagi, T., Nguyen, D.L., Lancelot, C., Lamonier, C., Thavornprasert, K.A., Wenli, Z., Capron, M., Jalowiecki-Duhamel, L., Umbarkar, S., Dongare, M., Dumeignil, F.: Transesterification of diethyl oxalate with phenol over sol-gel MoO3/TiO2 catalysts. Chemsuschem 5, 1467–1473 (2012). https://doi.org/10.1002/cssc.201100802
Cui, L.P., Li, Y.J., Li, Z., Zhao, J.F.: MoO3/SO42–TiO2 catalyst for transesterification of dimethyl cabonate with phenol. J. Cent. South Univ. 21, 1719–1724 (2014). https://doi.org/10.1007/s11771-014-2115-0
Acknowledgement
We are grateful to CHRIST (Deemed to be University), Bangalore for all the facilities and support. We are thankful to Bangalore Institute of Technology and Indian Institute of Science (IISc) Bangalore for various characterization facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Priya, A.S., Devi, K.R.S., Karthik, K. et al. Modified Rice Husk Silica from Biowaste: An Efficient Catalyst for Transesterification of Diethyl Malonate and Benzyl Alcohol. Waste Biomass Valor 11, 4809–4819 (2020). https://doi.org/10.1007/s12649-019-00808-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-019-00808-5