Abstract
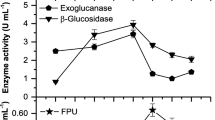
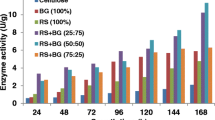
Lignocellulosic material is an alternative, renewable and cheaper source of molecules to be applied in greener industrial processes. Its utilization for this purpose requests steps of pre-treatment and hydrolysis. Filamentous fungi are receiving attention as source of plant cell wall degrading enzymes to apply in lignocellulosic biomass hydrolysis. In the present study, a strain of Penicillium chrysogenum CCDCA10756 isolated from Brazilian Cerrado soil (Savannah like biome) was evaluated as a producer of plant cell wall degrading enzymes aiming industrial application. The fungus cultivated in the presence of corn straw as sole carbon source secreted cellulases (endo-β-1,4-glucanases, cellobiohydrolases, β-glucosidases), endo-β-1,4-xylanases, and pectinases. Endo-β-1,4-xylanases and pectinases presented earlier production reaching maximum values after 3 days of growth in comparison to cellulolytic activities mostly produced after 5 days. Cellobiohydrolases and endo-β-1,4-glucanases present maximal activity in acid pH (3 and 4) and at 50 °C, whereas β-glucosidase presents maximal activity at pH 5.0 and 60 °C. Pectinases showed maximum activity in pH 8 at 50 °C. Furthermore, endo-β-1,4-glucanases and cellobiohydrolases displayed remarkable thermostability at 40 °C. Lignin-derived compounds, trans-ferulic acid, 4-hydroxybenzoic and syringaldehyde inhibited cellobiohydrolases. Pectinolytic activity, instead, was improved in the presence of p-coumaric acid, trans-ferulic acid, and syringaldehyde.







Similar content being viewed by others
References
Limayem, A., Ricke, S.C.: Lignocellulosic biomass for bioethanol production: current perspectives, potential issues and future prospects. Prog. Energy Combust. Sci. 38, 449–467 (2012). https://doi.org/10.1016/j.pecs.2012.03.002
Ferreira-Leitao, V., Gottschalk, L.M.F., Ferrara, M.A., Nepomuceno, A.L., Molinari, H.B.C., Bon, E.P.S.: Biomass residues in Brazil: Availability and potential uses. Waste Biomass Valoriz. 1, 65–76 (2010). https://doi.org/10.1007/s12649-010-9008-8
Soccol C.R., Vandenberghe L.P., Medeiros A.B., Karp S.G., Buckeridge M, Ramos L.P., Pitarelo A.P., Ferreira-Leitão V, Gottschalk L.M., Ferrara MA, da Silva Bon E.P., de Moraes L.M., Araújo Jde A, Torres F.A.: Bioethanol from lignocelluloses: Status and perspectives in Brazil. Bioresour. Technol. 101, 4820–4825 (2010). https://doi.org/10.1016/j.biortech.2009.11.067
Demain, A.L.: Biosolutions to the energy problem. J. Ind. Microbiol. Biotechnol. 36, 319–332 (2009). https://doi.org/10.1007/s10295-008-0521-8
Strassberger, Z., Tanase, S., Rothenberg, G.: The pros and cons of lignin valorisation in an integrated biore fi nery. RSC Adv. 25310–25318 (2014). https://doi.org/10.1039/c4ra04747h
Saha, B.C.: Hemicellulose bioconversion. J. Ind. Microbiol. Biotechnol. 30, 279–291 (2003). https://doi.org/10.1007/s10295-003-0049-x
Anwar, Z., Gulfraz, M., Irshad, M.: Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: a brief review. J. Radiat. Res. Appl. Sci. 7, 163–173 (2014). https://doi.org/10.1016/j.jrras.2014.02.003
Singhania, R.R., Sukumaran, R.K., Patel, A.K., Larroche, C., Pandey, A.: Advancement and comparative profiles in the production technologies using solid-state and submerged fermentation for microbial cellulases. Enzyme Microb. Technol. 46, 541–549 (2010). https://doi.org/10.1016/j.enzmictec.2010.03.010
Gilbert, H.J., Hazlewood, G.P.: Bacterial cellulases and xylanases. J. Gen. Microbiol. 139, 187–194 (1993). https://doi.org/10.1099/00221287-139-2-187
Beg, Q.K., Kapoor, M., Mahajan, L., Hoondal, G.S.: Microbial xylanases and their industrial applications: a review. Appl. Microbiol. Biotechnol. 56, 326–338 (2001). https://doi.org/10.1007/s002530100704
Polizeli, M.L.T.M., Rizzatti, A.C.S., Monti, R., Terenzi, H.F., Jorge, J.A., Amorim, D.S.: Xylanases from fungi: properties and industrial applications. Appl. Microbiol. Biotechnol. 67, 577–591 (2005). https://doi.org/10.1007/s00253-005-1904-7
Jayani, R.S., Saxena, S., Gupta, R.: Microbial pectinolytic enzymes: a review. Process Biochem. 40, 2931–2944 (2005). https://doi.org/10.1016/j.procbio.2005.03.026
Kashyap, D.R., Vohra, P.K., Chopra, S., Tewari, R.: Applications of pectinases in the commercial sector: a review. Bioresour. Technol. 77, 215–227 (2001). https://doi.org/10.1016/S0960-8524(00)00118-8
Adeleke, A.J., Odunfa, S.A., Olanbiwonninu, A., Owoseni, M.C.: Production of cellulase and pectinase from orange peels by fungi\n. Nat. Sci. 10, 107–112 (2012)
Forsberg, Z., Mackenzie, A.K., Sørlie, M., Røhr, ÅK., Helland, R., Arvai, A.S., Vaaje-Kolstad, G., Eijsink, V.G.H.: Structural and functional characterization of a conserved pair of bacterial cellulose-oxidizing lytic polysaccharide monooxygenases. Proc. Natl. Acad. Sci. USA. 111, 8446–8451 (2014). https://doi.org/10.1073/pnas.1402771111
Bourbonnais, R., Paice, M.G., Reid, I.D., Lanthier, P., Yaguchi, M.: Lignin oxidation by laccase isozymes from Trametes versicolor and role of the mediator 2, 2’-azinobis (3-ethylbenzthiazoline-6-sulfonate) in kraft lignin depolymerization. Appl. Environ. Microbiol. 61, 1876–1880 (1995)
Ibrahim, V., Mendoza, L., Mamo, G., Hatti-Kaul, R.: Blue laccase from Galerina sp.: properties and potential for kraft lignin demethylation. Process Biochem. 46, 379–384 (2011). https://doi.org/10.1016/j.procbio.2010.07.013
Saloheimo, M., Paloheimo, M., Hakola, S., Pere, J., Swanson, B., Nyyssönen, E., Bhatia, A., Ward, M., Penttilä, M.: Swollenin, a Trichoderma reesei protein with sequence similarity to the plant expansins, exhibits disruption activity on cellulosic materials. Eur. J. Biochem. 269, 4202–4211 (2002). https://doi.org/10.1046/j.1432-1033.2002.03095.x
Ertan, F., Balkan, B., Balkan, S., Aktac, T.: Solid state fermentation for the production of α -amylase from Penicillium chrysogenum using mixed agricultural by-products as substrate. 657–661 (2006). https://doi.org/10.2478/s11756-006-0137-2
Ferrer, M., Plou, F.J., Nuero, O.M., Reyes, F., Ballesteros, A.: Purification and properties of a lipase from Penicillium chrysogenum isolated from industrial wastes. J. Chem. Technol. Biotechnol. 75, 569–576 (2000). https://doi.org/10.1002/1097-4660(200007)75:7%3C569::AID-JCTB258%3E3.0.CO;2-S
Nwodo, S.C., Uzoma, A.O., Thompson, N.E., Victoria, I.O.: Xylanase production of Aspergillus niger and Penicillium chrysogenum from ammonia pretreated cellulosic waste. Res. J. Microbiol. 3(4), 246–253 (2008)
Vangulik, W.M., Antoniewicz, M.R., Delaat, W.T.A.M., Vinke, J.L., Heijnen, J.J.: Energetics of growth and penicillin production in a high-producing strain of Penicillium chrysogenum. Biotechnol. Bioeng. 72, 185–193 (2001). https://doi.org/10.1002/1097-0290(20000120)72:2%3C185::AID-BIT7%3E3.0.CO;2-M
Backus, M.P., Stauffer, J.F., Johnson, M.J.: Penicillin yields from new mold strains. J. Am. Chem. Soc. 68, 152–153 (1946). https://doi.org/10.1021/ja01205a518
Yang, Y., Yang, J., Liu, J., Wang, R., Liu, L., Wang, F., Yuan, H.: The composition of accessory enzymes of Penicillium chrysogenum P33 revealed by secretome and synergistic effects with commercial cellulase on lignocellulose hydrolysis. Bioresour. Technol. 257, 54–61 (2018). https://doi.org/10.1016/j.biortech.2018.02.028
Duarte, G., Moreira, L., Gómez-Mendoza, D., Siqueira, F.G., De Batista, L., Amaral, L., Ricart, C., Filho, E.: Use of Residual biomass from the textile industry as carbon source for production of a low-molecular-weight xylanase from Aspergillus oryzae. Appl. Sci. 2, 754–772 (2012). https://doi.org/10.3390/app2040754
Miller, G.L.: Use of dinitrosaiicyiic acid reagent for determination of reducing sugar. Anal. Chem. 3, 426–428 (1959)
Bradford, M.M.: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976). https://doi.org/10.1016/0003-2697(76)90527-3
Ximenes, E., Kim, Y., Mosier, N., Dien, B., Ladisch, M.: Inhibition of cellulases by phenols. Enzyme Microb. Technol. 48, 54–60 (2011). https://doi.org/10.1016/j.enzmictec.2010.09.006
Ximenes, E., Kim, Y., Mosier, N., Dien, B., Ladisch, M.: Deactivation of cellulases by phenols. Enzyme Microb. Technol. 48, 54–60 (2011). https://doi.org/10.1016/j.enzmictec.2010.09.006
De S. Moreira L.R., De Carvalho Campos, M., De Siqueira, P.H., Silva, L.P., Ricart, C.A., Martins, P.A., Queiroz, R.M., Filho, E.X..: Two β-xylanases from Aspergillus terreus: characterization and influence of phenolic compounds on xylanase activity. Fungal Genet. Biol. 60, 46–52 (2013). https://doi.org/10.1016/j.fgb.2013.07.006
LAEMMLI, U.K.: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227, 680–685 (1970)
Zhang, M., Su, R., Qi, W., He, Z.: Enhanced Enzymatic Hydrolysis of Lignocellulose by Optimizing Enzyme Complexes. 1407–1414 (2010). https://doi.org/10.1007/s12010-009-8602-3
Goyal, A., Ghosh, B., Eveleigh, D.: Characteristics of fungal cellulases. Bioresour. Technol. 36, 37–50 (1991). https://doi.org/10.1016/0960-8524(91)90098-5
Haas, H., Herfurth, E., Stöffler, G., Redl, B.: Purification, characterization and partial amino acid sequences of a xylanase produced by Penicillium chrysogenum. Biochim. Biophys. Acta—Gen. Subj. 1117, 279–286 (1992). https://doi.org/10.1016/0304-4165(92)90025-P
Chen, M., Qin, Y., Liu, Z., Liu, K., Wang, F., Qu, Y.: Enzyme and microbial technology isolation and characterization of a  -glucosidase from Penicillium decumbens and improving hydrolysis of corncob residue by using it as cellulase supplementation. Enzyme Microb. Technol. 46, 444–449 (2010). https://doi.org/10.1016/j.enzmictec.2010.01.008
Krogh, K.B.R.M., Harris, P.V., Olsen, C.L., Johansen, K.S., Hojer-pedersen, J., Borjesson, J., Olsson, L.: Characterization and kinetic analysis of a thermostable GH3 β -glucosidase from Penicillium brasilianum. 143–154 (2010). https://doi.org/10.1007/s00253-009-2181-7
Terrone, C.C., Freitas, C., De, Rafael, C., Terrasan, F., Almeida, A.F., De Carmona, E.C.: Agroindustrial biomass for xylanase production by Penicillium chrysogenum: purification, biochemical properties and hydrolysis of hemicelluloses. Electron. J. Biotechnol. 33, 1–7 (2018). https://doi.org/10.1016/j.ejbt.2018.04.001
Sakamoto, T., Kawasaki, H.: Purification and properties of two type-B a-L-arabinofuranosidases produced by Penicillium chrysogenum. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1621, 204–210 (2003). https://doi.org/10.1016/S0304-4165(03)00058-8
Hoondal, G., Tiwari, R., Tewari, R., Dahiya, N., Beg, Q.: Microbial alkaline pectinases and their industrial applications: a review. Appl. Microbiol. Biotechnol. 59, 409–418 (2002). https://doi.org/10.1007/s00253-002-1061-1
Alafia, A., Llama, M.J.: Purification and some properties of the pectin lyase from Penicillhtm italicum. 2, 335–340 (1991)
Narra, M., Dixit, G., Divecha, J., Kumar, K., Madamwar, D., Shah, A.R.: Production, purification and characterization of a novel GH 12 family endoglucanase from Aspergillus terreus and its application in enzymatic degradation of delignified rice straw. Int. Biodeterior. Biodegrad. 88, 150–161 (2014). https://doi.org/10.1016/j.ibiod.2013.12.016
Johnson, E.A., Demain, A.L.: Probable involvement of sulfhydryl groups and a metal as essential components of the cellulase of Clostridium thermocellum. Arch. Microbiol. 137, 135–138 (1984)
Akiba, S., Kimura, Y., Yamamoto, K., Kumagai, H.: Purification and characterization of a protease-resistant cellulase from Aspergillus niger. J. Ferment. Bioeng. 79, 125–130 (1995)
Sposina, R., Teixeira, S., Souza, M.V., De Ximenes, E., Filho, F.: Purification and characterization studies of a thermostable b -xylanase from Aspergillus awamori. J. Ind. Microbiol. Biotechnol 37, 1041–1051 (2010). https://doi.org/10.1007/s10295-010-0751-4
Tejirian, A., Xu, F.: Inhibition of cellulase-catalyzed lignocellulosic hydrolysis by iron and oxidative metal ions and complexes. Appl. Environ. Microbiol. 76, 7673–7682 (2010). https://doi.org/10.1128/AEM.01376-10
Xu, F., Ding, H., Tejirian, A.: Detrimental effect of cellulose oxidation on cellulose hydrolysis by cellulase. Enzyme Microb. Technol. 45, 203–209 (2009). https://doi.org/10.1016/j.enzmictec.2009.06.002
Davies, G., Henrissat, B.: Structures and mechanisms of glycosyl hydrolases. Structure. 3, 853–859 (1995). https://doi.org/10.1016/S0969-2126(01)00220-9
Tejirian, A., Xu, F.: Inhibition of enzymatic cellulolysis by phenolic compounds. Enzyme Microb. Technol. 48, 239–247 (2011). https://doi.org/10.1016/j.enzmictec.2010.11.004
Yaoi, K., Kondo, H., Hiyoshi, A., Noro, N., Sugimoto, H., Tsuda, S., Mitsuishi, Y., Miyazaki, K.: The Structural basis for the exo-mode of Action in GH74 oligoxyloglucan reducing end-specific cellobiohydrolase. J. Mol. Biol. 370, 53–62 (2007). https://doi.org/10.1016/j.jmb.2007.04.035
Cannella, D., Hsieh, C.W.C., Felby, C., Jørgensen, H.: Production and effect of aldonic acids during enzymatic hydrolysis of lignocellulose at high dry matter content. Biotechnol. Biofuels. 5, 1–10 (2012). https://doi.org/10.1186/1754-6834-5-26
Vieira, W.B., Rios, L., Moreira, D.S., Neto, A.M., Ximenes, E., Filho, F.: Production and Characterization of an Enzyme Complex From a New Strain of Clostridium Thermocellum With Emphasis on Its Xylanase Activity. Braz. J. Microbiol. 38, 237–242 (2007)
Yu, P., Xu, C.: Production optimization, purification and characterization of a heat-tolerant acidic pectinase from Bacillus sp. ZJ1407. Int. J. Biol. Macromol. 108, 972–980 (2018). https://doi.org/10.1016/j.ijbiomac.2017.11.012
Monti, A., Di Virgilio, N., Venturi, G.: Mineral composition and ash content of six major energy crops. Biomass Bioenergy. 32, 216–223 (2008). https://doi.org/10.1016/j.biombioe.2007.09.012
Wu, H.S., Wang, Y., Zhang, C.Y., Bao, W., Ling, N., Liu, D.Y., Shen, Q.R.: Growth of in vitro Fusarium oxysporum f. sp. niveum in chemically defined media amended with gallic acid. Biol. Res. 42, 297–304 (2009). https://doi.org/10.4067/S0716-97602009000300004
Duarte, G.C., Moreira, L.R.S., Jaramillo, P.M.D., Filho, E.X.F.: Biomass-derived inhibitors of holocellulases. Bioenergy Res. 5, 768–777 (2012). https://doi.org/10.1007/s12155-012-9182-6
Silva, C., de O.G., Aquino, Ricart, E.N., Midorikawa, C.A.O., Miller, G.E.O., Filho, R.N.G.: E.X.F.: GH11 xylanase from emericella nidulans with low sensitivity to inhibition by ethanol and lignocellulose-derived phenolic compounds. FEMS Microbiol. Lett. 362, 1–8 (2015). https://doi.org/10.1093/femsle/fnv094
Acknowledgements
This work was supported by research grants from the University of Brasilia – UnB, CNPq, and FAPDF. Eliane F. Noronha is recipient of Brazilian Research Council (CNPq) research scholarship. Pedro R.V Hamann, and Alonso R.P. Ticona are recipient of CAPES doctoral degree scholarship, Sadia F. Ullah is the recipient of CNPq doctoral’s degree scholarship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Silva, L.d.M.B., Gomes, T.C., Ullah, S.F. et al. Biochemical Properties of Carbohydrate-Active Enzymes Synthesized by Penicillium chrysogenum Using Corn Straw as Carbon Source. Waste Biomass Valor 11, 2455–2466 (2020). https://doi.org/10.1007/s12649-019-00589-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-019-00589-x