Abstract
In the present work, dry plant biomass that is commonly used in horses feeding: alfalfa, chopped grass pellets and chopped grass was used as a biosorbent of microelement ions: Cr(III), Zn(II), Cu(II) and Mn(II). Biosorption process was used to potentially enhance supplementation of minerals in horses diet. In order to characterize the biosorption properties of the examined biomass, preliminary experiments (kinetics and equilibrium studies) were conducted with Cr(III) ions. The effect of pH and initial metal ions concentration (C0) on the biosorption capacity (q) was investigated. On this basis, the best experimental conditions were chosen (pH 5, CS 1.0 g/L (biomass content), C0 200 mg/L). For them, biosorption of Mn(II), Zn(II), Cu(II) ions was carried out in a periodic and continuous system (biosorption column). Equilibrium of the biosorption was described using Langmuir equation. The mineral composition of the raw and enriched biomass was also examined using ICP-OES, as well as SEM-EDX technique. In general, the best biosorption properties were observed for alfalfa. The maximum biosorption capacity for Zn(II) was 27.5 mg/g, for Cu(II)—38.5 mg/g, for Mn(II)—13.6 mg/g and for Cr(III)—33.2 mg/g. Therefore, the biomass of enriched alfalfa can be recommended as a feed supplement for horses.
Similar content being viewed by others
Statement of Novelty
In the present work, we proposed for the first time to use biomass, commonly used in horse feeding as a carrier of microelements, especially with a diagnosed metabolic syndrome. Alfalfa, chopped grass pellets and chopped grass were used as biosorbents of microelement ions such as Cr(III), Zn(II), Cu(II) and Mn(II). Biosorption was used to enhance supplementation of minerals in horse diet.
Introduction
Minerals play a crucial role in the horse diet although they constitute only a minor part of it. They are involved in many physiological processes and are integral parts of amino acids, hormones, vitamins [1]. In this paper we focused on copper, manganese, zinc and chromium which are required by horses in small quantities. Copper plays role in collagen stabilization, elastin syntheses, biomechanical properties of corneous processes of the skin and mobilization of iron stores and melanin syntheses [2]. Manganese is essential for carbohydrate and lipid metabolism, synthesis of chondroitin sulphate. It acts as a superoxide scavenger. Zinc is a component of metalloenzymes involved in protein and carbohydrate metabolism [1, 3]. Chromium is a component of glucose tolerance factor which potentates the action of insulin, as well as is involved in carbohydrate metabolism [4]. It’s biological action is thought to elicit its effects by enhancing intracellular insulin receptor signalling pathways through enhancing tyrosine kinase activity or reduced tyrosine phosphatase activity [5]. This in consequence might potentially increase insulin sensitivity [5, 6]. Although chromium has been reported to have a beneficial effect in diabetes mellitus patients, contrary data exist in the field of equine nutrition [5, 7, 8]. Chameroy and colleagues have questioned the beneficial effect of chromium supplementation on morphometric measurements, blood variables, resting insulin concentrations or insulin sensitivity in laminitic obese horses [9]. However, other authors suggested, that chromium might improve insulin sensitivity by altering the intracellular signalling pathways initiated by the activated insulin receptor [5]. The contrary data might be also due to the source of chromium or its chemical form which especially in equine nutrition still require further investigation.
The main source of minerals for horses are forages and concentrates. Their content in feed and availability depend on soil mineral concentration, plant species, stage of maturity and conditions of harvesting [1]. According to the Nutrient Requirements of Horses [1], the content of microelements in a feedstuff differs—for example for copper it ranges from 1 mg/kg for corn to 80 mg/kg for cane molasses, for zinc it is from 15 to 40 mg/kg, for manganese in forages from 40 to 140 mg/kg and most concentrates contain from 15 to 45 mg/kg. According to the guidelines of Pagan et al. [4], the recommend dose of chromium in horse diet is 5.0 mg/day. In order to prevent deficiencies of these elements, very often they are supplemented to the feed, mainly as inorganic salts—sulphates, chlorides, oxides, carbonates or less frequently organic-chelate. A crucial issue is the determination of oral bioavailability (which is usually low for inorganic salts) and appropriate dosages for mineral supplements used in horse feeding [9].
In this publication we propose to produce biological feed additives with microelements using biosorption process. This process may be defined as the binding of desired substances (e.g., metal ions) from aqueous solution by a biological material. Metal ions are bound with functional groups present on the surface of the cell wall [10]. The biosorption process can be performed using different types of low-cost biomass, that can be used in animal feeding [11, 12]. Here, we propose to apply traditionally used in equine feeding alfalfa, chopped grass pellets and chopped grass as a carrier of microelement ions—Cu(II), Zn(II), Mn(II) and Cr(III). In the literature the biomasses of alfalfa and grass are randomly studied as a biosorbent of metal ions. This biomass was rather treated as a biosorbent to remove metal ions such as Er(III), Ho(III), Ni(II), Pb(II), Cr(III) from wastewaters. Some of the examples are presented in Table 1.
In our previous studies we showed that biomass enriched with microelement ions constituted a source of highly bioavailable minerals to animals (laying hens, pigs, goats) and can partly replace traditionally used inorganic salts as a main source of microelements [20,21,22]. In these studies it was shown that the bioavailability of microelements from the enriched biomass was higher than from inorganic salts. The new feed additive produced from the biomass of alfalfa, chopped grass pellets and chopped grass additionally enriched with Cu(II), Zn(II), Mn(II) and Cr(III) can be used in horse feeding, especially with diagnosed equine metabolic syndrome (EMS) which is a serious endocrine disorder characterized by severe obesity, insulin resistance, systemic inflammation and past or chronic laminitis [23,24,25]. Most recently, herbal bioactive substances have been showed to possess a beneficial effect in EMS feeding through improvement of insulin sensitivity [26].
Dietary management in EMS horses involves feeding grass hay (restricted to 1.25% of body mass as daily dry matter intake) with the addition of vitamin and mineral supplements [27]. Hay with low non-structural carbohydrates (NSC; simple sugars, starch and fructan) content should be selected [25]. These non-structural carbohydrates can induce laminitis. They are digested rapidly and energy is released quickly for a short burst of energy [27, 28]. Additionally, soaking of hay is recommended because it results in a significant reduction in the content of these carbohydrates [27, 29]. Among mineral additives, especially chromium and magnesium are commonly recommended for the management of EMS [24, 25].
In the present paper we examined the biosorption properties of alfalfa, chopped grass pellets and chopped grass as a potential natural feed additives for horses with EMS. Preliminary experiments (kinetics and equilibrium studies) were conducted with Cr(III) ions in a batch system. The effect of pH and initial metal ions concentration (C0) on the biosorption capacity (q) was investigated. On this basis, the best experimental conditions were chosen. For them, biosorption of Mn(II), Zn(II), Cu(II) ions was carried out in a batch, as well as in a fixed-bed column system. The potential of the enriched biomass as a feed additive for horses with EMS was discussed.
Experimental
Materials
Chemicals
All reagents: Cr(NO3)3·9H2O, ZnSO4·7H2O, CuSO4·5H2O, MnSO4·H2O, NaOH, HCl, EDTA were purchased from POCh S.A. Gliwice, Poland.
Biosorbents
Three following feed materials, commonly used in horse feeding were used as biosorbents for metal ions:
-
1.
alfalfa (http://hippovetpl.shoper.pl/pl/p/Luzerne-Mix-20-kg/123) composed of young chopped alfalfa (50%), young chopped green oat (30%), clover (10%), sugar beet molasses (5%) and vegetable oils (5%).
-
2.
chopped grass pellets (http://hippovetpl.shoper.pl/pl/p/PreAlpin-Wiesencobs-25-kg/124) composed of unique mixture of over 60 different grasses and herbs of the Bavarian foothills of the Alps. The fields are under constant supervision and are harvested at the right time of maturation. This ensures low protein and a high fibre content for optimum horse nutrition.
-
3.
chopped grass (http://hippovetpl.shoper.pl/pl/p/PreAlpin-Aspero-20-kg/125)—a very special chaff/chop produced from a blend of 60 grasses and herbs from the foothills of the Bavarian Alps, lightly sprayed with cold pressed linseed (2% added).
Before the use in the biosorption studies, the listed raw biomass (Fig. 1) was finely ground using RETSCH Knife Mill Grindomix GM300 (Germany).
Their chemical composition provided by the Producer (HippoVet+ (Poland)) is presented in Table 2.
Biosorption Process
In order to compare biosorption properties of each tested biomass, biosorption capacity (qt; mg/g) was calculated as the difference between the initial (C0; mg/L) and final (Ct at time t; mg/L) concentration of metal ions in the solution divided by the concentration of this biomass in the solution (g/L). In other words, biosorption capacity is a mass of ions (mg) bound by the mass of the sorbent (g) [11].
Kinetic Studies
The aim of kinetic experiments was to establish the best experimental conditions of biosorption process (pH and the initial concentration of metal ions in the aqueous solution) and to establish time required to reach the process equilibrium. Additionally, pseudo-second order model (1) was used to determine biosorption capacity at equilibrium (qeq, mg/g) and k2 which is the rate constant of second-order biosorption (g/mg min).
Linear form of Eq. [1] is expressed as:
where qt is the amount of adsorbed metal ions on the biosorbent at time t (mg/g). The plot of t/qt against t (Eq. 2) gives a linear relationship, from which qeq and k2 can be determined. This model is the most often used for the description of biosorption of metal ions by biosorbents [30, 31].
For this reason, Cr(III) ions (as an inorganic salt Cr(NO3)3·9H2O) were chosen due to simple method of their determination in aqueous solutions—spectrophotometric method [32]. Kinetics experiments for biosorption were performed in Erlenmeyer flasks containing 200 mL of Cr(III) solution at agitating rate—150 rpm (IKA KS 260 basic) and room temperature (20 °C) for 3 h. The examined experimental conditions were as follows:
-
(1)
Effect of initial Cr(III) concentration (C0) on the biosorption capacity was examined for C0: 100, 200 and 300 mg/L (pH 5, biomass concentration (CS) 1.0 g/L);
-
(2)
Effect of pH on the biosorption capacity was examined for pH: 3, 4 and 5 (CS 1.0 g/L, C0 300 mg/L).
pH of the solutions was adjusted with 0.1 mol/L solution NaOH/HCl using pH meter Mettler-Toledo (Seven Multi; Greifensee, Switzerland) equipped with an electrode InLab413 with compensation of temperature. Detailed description of kinetic experiments can be found in the work of Michalak and Chojnacka [31].
Biosorption Equilibrium
The equilibrium studies were performed in order to determine from the Langmuir model [33] the maximum possible amount of metal ions sorbed per gram of biosorbent—qmax (mg/g) and the affinity of binding sites for the metal ions—b (L/mg) which is a Langmuir adsorption constant. These parameters were determined after linearization of Eq. [3]:
Linear form of Langmuir equation is expressed as:
where Ceq is concentration of metal ions in the solution in equilibrium (mg/L).
The essential characteristics and the feasibility of the Langmuir isotherm can be also expressed in terms of a dimensionless constant separation factor RL, which is defined as:
where C0 is the highest initial concentration of metal ions in the solution (mg/L) and b the Langmuir adsorption constant (L/mg). The RL value implies the adsorption to be unfavourable for RL > 1, linear for RL = 1, favourable for the range 0 < RL < 1 or irreversible for RL = 0 [34].
The equilibrium experiments were performed in Erlenmeyer flasks containing 20 mL of metal ions solution in a shaker at 150 rpm (detailed description in Michalak and Chojnacka, [35]). These experiments were carried out at the best process conditions (25 °C, pH 5, CS 1.0 g/L) determined in the kinetic experiments. The initial concentrations of Cr(III), Zn(II), Cu(II) and Mn(II) ions in the solutions were as follows: 10, 25, 50, 75, 100, 150, 200, 250 and 300 mg/L. pH each of them was 5. For the preparation of metal ion solutions, the following inorganic salts were used: ZnSO4·7H2O, CuSO4·5H2O, MnSO4·H2O. The contact time was 2.5 h (determined from the kinetic experiments).
Biosorption in a Fixed-Bed Column
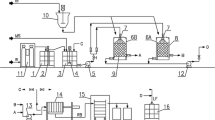
The aim of this experiment was to enrich the biomass with microelements in the column system to obtain feed additive for horses. This system is known to be more effective (amount of the enriched biomass production) than the batch system. The examined biomasses (20 g) were stacked separately into glass columns with the volume of 0.5 L. The microelements bearing solutions (C0 250 mg/L, pH 5) were continuously pumped upward into the column. The flow rate was 1 mL/min. The process lasted for 4 h. After the process, the enriched biomass was dried and subjected to the ICP-OES and SEM-EDX analysis. It allowed to characterize the biomass (chemical composition), as well as to examine the mechanism of the biosorption process.
Analytical Methods
Spectrophotometric Method
The concentration of Cr(III) ions in the solution (4 mL) before and after biosorption process was determined spectrophotometrically (λ 540 nm) by complexation with EDTA (thylenediaminetetraacetic acid; 0.095 g) using Varian Cary 50 Conc. Instrument (Victoria, Australia). EDTA reacts with Cr(III) and forms a stable, violet-coloured complex at higher temperatures ~ 95 °C. Detailed description of the methodology can be found in the work of Michalak and Chojnacka [31] and Ni et al. [32].
Inductively Coupled Plasma-Optical Emission Spectrometry
The concentrations of Cu(II), Mn(II), Zn(II) in the solutions before and after biosorption process (equilibrium studies), as well as the content of micro- and macroalements in the biomass enriched in a fixed-bed column system were determined by ICP-OES technique (inductively coupled plasma optical emission spectrometry) using Varian VISTA-MPX ICP-OES spectrometer (Victoria, Australia) in the Chemical Laboratory of Multielemental Analysis at Wrocław University of Science and Technology accredited by International Laboratory Accreditation Cooperation Mutual Recognition Arrangement and Polish Centre for Accreditation. Before the analysis, enriched biomass (about 0.5 g) was digested with 5 mL of concentrated 69% nitric acid (Supra pure grade from Merck, Darmstadt, Germany) in Teflon bombs in a microwave oven (type Milestone MLS-1200 MEGA, Bergamo, Italy). The solution after mineralization was diluted to 50 mL and analysed by ICP-OES [20, 31].
Scanning Electron Microscopy with an Energy Dispersive X-ray Analytical System
Scanning electron microscopy analysis combined with multielemental X-ray analysis of each biosorbent before and after biosorption was performed at Wrocław University of Environmental and Life Sciences. Samples of the examined biosorbents were dehydrated in alcohol series, dried, placed on an appropriate stub and gold-sputtered (using Scancoat 6 equipment—Oxford). All samples were analysed using a EVO Zeiss LS15 (Oberkochen, Germany), operating at 20 kV. The X-ray analysis were carried out with application of Rontec system (Bruker, Germany) according to previously described method [36,37,38].
Results and Discussion
In the present study we examined biosorption properties of three biomasses that are used in horse feeding. For this reason, alfalfa, chopped grass pellets and chopped grass were chosen. In the literature, this biomass is considered as a low-cost biosorbent used for the removal of toxic metals, for example alfalfa—biosorption of Er(III) and Ho(III) [13], Ni(II) and Pb(II) [14]; Cd(II), Cr(III), Cr(VI), Pb(II), Zn(II) [15]. Grass as a biosorbent was used for the removal of Cr(III) [16, 17]; Cd(II) [17]; Cu(II) [18], Pb(II) and Ni(II) [19] (Table 1). Some of the listed metal ions, for example Cr(III), Zn(II), Cu(II) in small quantities are microelements that are required in horse feeding [1]. Therefore, the biomass of alfalfa and grass can be used to bind these metal ions via biosorption. Enriched biomass can then serve as a feed additive with microelements. Moreover, the chemical composition of the tested alfalfa and grass is suitable for horses with EMS, as it is recommended by their producer (Table 2). Therefore, the biomass enriched with mineral ions beside nutritional value can help in the management of EMS in horses.
Kinetic Studies
In this paper, the effect of pH and initial metal ions concentration on the parameters of pseudo-second order model were examined and the obtained data are presented in Table 3.
In the kinetic studies it was shown that the biosorption process was relatively fast—equilibrium was reached in around 80–100 min. Based on our previous studies, we examined the effect of pH (Fig. 2) for C0 300 mg of Cr(III) ions/L, room temperature and CS 1 g/L. Previously it was found that for higher concentrations of metal ions (> 300 mg/L) their amount bound with the biomass did not increase significantly. The difference in biosorption capacity of green macroalga—Enteromopha prolifera towards Cr(III) ions between 300 and 400 mg/L was small—only by 7% [31]. The aim of these experiments was to choose the optimal C0 in order to obtain the biomass enriched in the highest extent with the metal ion (taking into consideration economical aspects related to the prices of inorganic salts used for the preparation of aqueous solutions with different concentrations of metal ions).
Metal biosorption is fully dependent on pH of water [17, 18, 31, 39] and it was also found in our study. At low pH, the concentration of hydrogen ions (H+) increases and they compete with metal ions in the solution toward active sites on the surface of the biomass, leading to the lower biosorption capacity [19, 31].
The biosorption capacity of the biosorbents increased with pH—the highest qeq was found for pH 5. At pH 5 the biosorption capacity for alfalfa and chopped grass pellets was about two times higher than in pH 4 and about 3 times higher than in pH 3. For chopped grass qeq at pH 5 was about two times higher than at pH 4 and 3. When the initial pH of the solution was adjusted to a value higher than 5.5, chromium(III) ions precipitated as Cr(OH)3, because of higher concentration of OH− ions in the biosorption system [40].
Among three tested biosorbents, the highest qeq at pH 5 was for alfalfa which was 22% higher than for chopped grass pellets and 55% higher than for chopped grass. The same tendency concerning the effect of pH on the sorption of Cr(III) ions by grass was observed by Chojnacka [16]—qeq increased with pH: pH 3—qeq 7.15 mg/g; pH 4—qeq 7.31 mg/g and pH 5—qeq 14.1 mg/g (C0 200 mg/L, 20 °C, CS 5.0 g/L). In the work of Sulaymon et al. [17] it was found that the best pH value for Cr(III) removal by garden grass was around 4 and qeq was about 20 mg/g. Additionally, in the present paper it was shown that the rate constant of second-order model of biosorption, for all biosorbents, increased with pH.
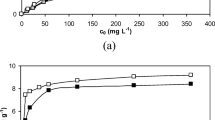
In the case of initial metal ions concentration (100, 200 and 300 mg/L) the highest qeq for all biosorbents was determined for C0 200 mg/L (Fig. 3). This can indicate that at higher concentrations (C0 > 200 mg/L), the available sites for biosorption became fewer and the saturation of the sorption sites can be observed [17, 31]. In the case of alfalfa, qeq in 200 mg/L was 4.5% higher than in 300 mg/L; for chopped grass pellets by 11% and for chopped grass by 24%. These results are consistent with the data presented by Chojnacka [16], where qeq for 200 mg/L was 14.1 mg/g and for 300 mg/L was 14.3 mg/g (20 °C, pH 5.0, CS 5.0 g/L). Among tested biosorbents, the best biosorption properties in these experimental conditions (pH 5, C0 200 mg/L, room temperature, CS 1 g/L) showed again alfalfa—qeq was 14% higher than for chopped grass pellets and 30% higher than for chopped grass. The presented results showed that the value k2 for the examined biosorbents did not depend on the initial concentration of Cr(III) ions.
Biosorption Equilibrium
The model parameters of Langmuir equation and dimensionless constant separation factor RL are presented in Table 4.
The maximum biosorption capacity determined from Langmuir equation was similar for all biosorbents towards Zn(II) and Cr(III) ions. The mean qmax was 28.2 ± 0.7 mg/g for Zn(II) ions and 31.6 ± 2.2 mg/g for Cr(III) ions. In the case of Cu(II) and Mn(II) ions, mean qmax of alfalfa and chopped grass pellets was comparable and equal to 38.0 ± 0.7 for Cu(II) ions and 14.4 ± 1.1 mg/g for Mn(II) ions. The maximum biosorption capacity of chopped grass towards Cu(II) ions was by 37% lower than mean qmax for alfalfa and chopped grass pellets. For Mn(II) ions, qmax of chopped grass was more than two times higher than for alfalfa and chopped grass pellets. The order of qmax determined from Langmuir equation was the same for alfalfa and chopped grass pellets: Cu > Cr > Zn > Mn. In the case of chopped grass, the order was as follows: Cr > Mn > Zn > Cu. Biosorption isotherms are shown in Fig. 4.
The values of RL for biosorption of all tested metal ions by the examined biosorbents were in the range 0 < RL < 1 what implies the adsorption to be favourable. Similar results were obtained by Sulaymon et al. [17] who examined the biosorption of Cd(II) and Cr(III) ions by garden grass. For both metal ions the values of RL were in a range from 0 to 1, indicating a favourable biosorption for both the metals.
The literature data concerning biosorption of microelement ions by alfalfa, chopped grass pellets and chopped grass are missing. Hossain et al. [18] examined biosorption of Cu(II) ions by garden grass at different initial concentrations (1–500 mg/L) of copper without adjusting pH. The highest qmax was obtained for CS 10 g/L—58.6 mg/g, then for 5 g/L—46.2 mg/g and finally for 0.5 g/L—26.2 mg/g. However it is hard to compare these results, because as it was shown in the kinetic experiments, pH is the main factor that influences the biosorption capacity.
Biosorption in a Fixed-Bed Column
The data obtained for biosorption under batch conditions are generally not applicable to most industrial processes, for example wastewater treatment, where column operations dominate. Biosorption in a batch system is used mainly to determine the best experimental conditions for column operations [39]. For the production of new biosorption-based preparations with microelements it is necessary to scale up the process from the laboratory to the larger scale (industrial). Samoraj et al. [41] proposed a new plant for biosorption which is equipped with two independent reactors (70 L each) that can operate as a stirred tank or as a fixed-bed column system. It was shown that the daily productivity was 300–400 g in the first option and 10–16 kg in the second one, which is more effective. The multielemental composition of the examined biomasses enriched with microelement ions in a fixed-bed columns in the laboratory scale is presented in Table 5.
This enrichment was performed in the best experimental conditions determined from kinetic studies. It was shown that the amount of metal ions bound by the biomass in a column system was as follows: for alfalfa—2.6 mg/g for Zn, 2.0 mg/g for Cu, 1.6 mg/g for Mn and 2.1 mg/g for Cr. For chopped grass pellets these values were as follows: 3.0 mg/g for Zn, 3.4 mg/g for Cu, 2.2 mg/g for Mn and 2.1 mg/g for Cr. In the case of chopped grass, we obtained: 2.7 mg/g for Zn, 3.5 mg/g for Cu, 1.8 mg/g for Mn and for Cr. Results obtained in this study show that it was possible to significantly increase the content of a given microelement in the tested biomass. For example, in alfalfa the content of Cr in the enriched biomass increased 549 times when compared with natural biomass, content of Cu 321 times, content of Mn 90 times and content of Zn 158 times. Additionally from these results it can be seen that one of the mechanisms of biosorption process is ion exchange, in which alkali and alkaline earth metal ions (e.g., K, Ca, Mg) bound with functional groups (mainly carboxyl, hydroxyl) on the surface of the biomass are exchanged with metal ions from aqueous solution [11, 39]. In the present study, the dominating metal ion that was exchanged was K and Mg. Taking into account practical and economical point of view, biosorption in column is regarded as a more efficient method than in a batch system [15].
SEM Micrographs of Natural and Enriched Biomass
SEM-EDX technique gives the possibility to analyse the surface composition of the biomass before and after biosortion process. This method is used for the identification of elements visible only on the surface of biomass, as well as for observation of morphological changes in the biomass, e.g. shrinking after modifications [37]. The multielemental analysis of the examined biomass i.e. alfalfa, chopped grass pellet and chopped grass using SEM-EDX technique, as well as the SEM images are presented in Figs. 5, 6 and 7. It was shown, that the biosorption of Cr, Cu, Mn and Zn ion by alfalfa resulted in the final biomass enrichment with Cr equal to 0.71 wt% (weight percentage), 0.94, 1.16 and 0.97 wt% respectively. In turn, chopped grass pellet enriched with Cr, Cu, Mn and Zn ions resulted in the final biomass enrichment in Cr equal to 0.88, 1.54, 0.90 and 0.86 wt% respectively. Chopped grass enriched with Cr, Cu, Mn and Zn ion exhibited following final biomass enrichment: Cr 1.02 wt%, Cu—non detected, Mn—1.46 wt% and Zn—0.36 wt%. Performed analysis clearly showed that biosorption process resulted in the biomass enrichment when compared to the control biomass—not enriched with particular elements.
Taking into account SEM-EDX analysis it was shown that the highest amounts of Cr and Mn was observed in case of chopped grass surface, Cu for chopped grass pellet and Zn for alfalfa. Both, SEM-EDX and ICP-OES techniques confirmed that biosorption is a surface phenomenon, in which alkali and alkaline earth metal ions (e.g., Na, K, Ca, Mg) were exchanged by metal ions from aqueous solution [37, 39].
The Enriched Biomass in the Supplementation of Horses Diet
Minerals are particularly important for the health of horses. They play a significant role in a wide range of biochemical systems that affect every metabolic function in the horse [3]. In the present paper, a special attention was paid to manganese, copper, zinc and chromium. The NRC requirements for horses for the examined minerals are as follows: Mn 40 mg/kg, Cu 10 mg/kg, Zn 40 mg/kg (content in total diet, dry mass) [1]. The National Research Council does not have a specific recommendation for chromium. According to the guidelines of Pagan et al. [4], the recommend dose of chromium is 5 mg/day. In order to cover this requirement for these minerals, the enriched biomass can be added to the feed instead of inorganic salts. This biomass would carry a biologically bound, concentrated form of microelements to the horse diet. Therefore the amounts added to the feed will be reduced, what is also beneficial taking into account the environmental aspect. For example, for alfalfa (taking into account the maximum biosorption capacity) the amount of the enriched biomass which should be added to 1 kg of feed will be: 1.5 g for Zn, 0.26 g for Cu, 2.9 g for Mn and 0.15 g for Cr. The literature data concerning supplementation of chromium in horse diet are contradictory. Pagan et al. [4] showed that supplementation of chromium in the form of chromium yeasts to the trained horse diet influenced the levels of plasma cortisol, glucose and insulin which were lower than in the control group. Chameroy et al. [9] showed that the supplementation of horse diet (laminitic obese horses) with chromium yeast (5 mg/day) did not alter morphometric measurements (bodyweight, body condition, girth circumference, and neck circumference), blood variables, resting insulin concentrations or insulin sensitivity.
Conclusions
In the present paper, biosorption process of microelement ions by feedstuff components was used to produce concentrated form of elements for horses. For this reason, alfalfa, chopped grass pellets and chopped grass were chosen, since they are commonly used in horse feeding. The biosorption properties of the biomass were examined in kinetic and equilibrium experiments. On this basis, the best experimental conditions (pH 5, C0 200 mg/L) were chosen from biosorption kinetics, as well as the maximum biosorption capacity for each metal ion from Langmuir equation was determined. In the case of Zn(II) ions, the maximum biosorption capacity was comparable for all biosorbents—alfalfa, chopped grass pellets and chopped grass. For copper, the highest qmax was determined for alfalfa which was higher by 3% when compared with chopped grass pellets and by 37.5% higher than for chopped grass. Chopped grass was a good biosorbent of Mn(II) ions—qmax was more than two times higher than in the case of alfalfa and chopped grass pellets. The maximum biosorption capacity of alfalfa towards Cr(III) ions was the highest—33.22 mg/g, when compared with chopped grass pellets—29.15 mg/g and chopped grass—32.57 mg/g. Concluding, among the tested biomasses, the best biosorption properties had alfalfa. In the next step, the biomass was enriched in a column system and then analysed using ICP-OES and SEM-EDX techniques. Both methods confirmed binding of metal ions by the biomass—the first one in the whole biomass, the second one on the surface of the samples. Taking into account the maximum biosorption capacity of the biosorbents, the amount of the enriched biomass which should be added to 1 kg of feed to cover the requirement of horses for a given element was calculated. For the best biosorbent—alfalfa these values are as follows: 1.5 g of the biomass enriched with Zn, 0.26 g with Cu, 2.9 g with Mn and 0.15 g with Cr. The enriched alfalfa can be recommended as a feed additive for horses. It will provide microelement ions in a highly bioavailable form, however it should be confirmed in the in vivo experiments.
References
NRC (2007) The Nutrient Requirements of Horses, 6th edn. National Academy Press, Washington DC.
Kania, M., Mikolajewska, D., Marycz, K., Kobielarz, M.: Effect of diet on mechanical properties of horse’s hair. Acta Bioeng. Biomech. 11(3), 53–57 (2009)
Crandell, K.: (2003) Vitamin and mineral requirements in the horse. In: Zimmermann, N.G. (ed), Proceedings of the 50th Maryland Nutrition Conference for Feed Manufacturers and 1st Mid-Atlantic Nutrition Conference, March 27–28, 2003, University of Maryland, Maryland Feed Industry Council, College Park, USA, pp. 195–202
Pagan, J.D., Jackson, S.G., Duren, S.E.: The effect of chromium supplementation on metabolic response to exercise in thoroughbred horses. In: Pagan, J.D. (ed.) Advances in Equine Nutrition, pp. 263–270. Nottingham University Press, Nottingham (1998)
Hummel, M., Standl, E., Schnell, O.: Chromium in metabolic and cardiovascular disease. Horm. Metab. Res. 39, 743–751 (2007). https://doi.org/10.1055/s-2007-985847
Vincent, J.B.: The biochemistry of chromium. J. Nutr. 130, 715–718 (2000). https://doi.org/10.1093/jn/130.4.715
Jeejeebhoy, K.N.: Chromium and parenteral nutrition. J. Trace Elem. Exp. Med. 12, 85–89 (1999)
Kim, D.S., Kim, T.W., Park, I.K., Kang, J.S., Om, A.S.: Effects of chromium picolinate supplementation on insulin sensitivity, serum lipids, and body weight in dexamethasone-treated rats. Metabolism 51(5), 589–594 (2002). https://doi.org/10.1053/meta.2002.31985
Chameroy, K.A., Frank, N., Elliott, S.B., Boston, R.C.: Effects of a supplement containing chromium and magnesium on morphometric measurements, resting glucose, insulin concentrations and insulin sensitivity in laminitic obese horses. Equine Vet. J. 43(4), 494–499 (2011). https://doi.org/10.1111/j.2042-3306.2010.00302.x
Davis, T.A., Volesky, B., Mucci, A.: A review of the biochemistry of heavy metal biosorption by brown algae. Water Res. 37, 4311–4330 (2003). https://doi.org/10.1016/S0043-1354(03)00293-8
Michalak, I., Chojnacka, K., Witek-Krowiak, A.: State of the art for the biosorption process—a review. Appl. Biochem. Biotechnol. 170, 1389–1416 (2013). https://doi.org/10.1007/s12010-013-0269-0
Michalak, I., Witek-Krowiak, A., Chojnacka, K., Bhatnagar, A.: Advances in biosorption of microelements—the starting point for the production of new agrochemicals. Rev. Inorg. Chem. 35(3), 115–133 (2015). https://doi.org/10.1515/revic-2015-0003
Gardea-Torresdey, J.L., Tiemann, K.J., Peralta-Videa, J.R., Parsons, J.G., Delgado, M.: Binding of erbium(III) and holmium(III) to native and chemically modified alfalfa biomass: a spectroscopic investigation. Microchem. J. 76, 65–76 (2004). https://doi.org/10.1016/j.microc.2003.10.012
Mosavi, S.B., Karimi, S., Feiziasl, V.: Biosorption of lead and nickel by Medicago sativa (alfalfa) and Datura from contaminated solution. Asian J. Chem. 22(3), 1700–1704 (2010)
Gardea-Torresdey, J.L., Gonzalez, J.H., Tiemann, K.J., Rodriguez, O., Gamez, G.: Phytofiltration of hazardous cadmium, chromium, lead and zinc ions by biomass of Medicago sativa (Alfalfa). J. Hazard Mater. 57(1–3), 290–293 (1998). https://doi.org/10.1016/S0304-3894(97)00072-1
Chojnacka, K.: Biosorption of Cr(III) ions by wheat straw and grass: a systematic characterization of new biosorbents. Pol. J. Environ. Stud. 15(6), 845–852 (2006)
Sulaymon, A.H., Mohammed, A.A., Al-Musawi, T.J.: Comparative study of removal of cadmium (II) and chromium (III) ions from aqueous solution using low-cost biosorbent. Int. J. Chem. Reactor Eng. 12(1), 477–486 (2014). https://doi.org/10.1515/ijcre-2014-0024
Hossain, M.A., Ngo, H.H., Guo, W.S., Setiadi, T.: Adsorption and desorption of copper(II) ions onto garden grass. Bioresour. Technol. 121, 386–395 (2012). https://doi.org/10.1016/j.biortech.2012.06.119
Al Hamouz, O.C.S., Amayreh, M.Y.: Removal of lead(II) and nickel(II) ions from aqueous solution via Bermuda grass biomass. J. Water Supply: Res. Technol.-AQUA. 65(6), 494–503 (2016). https://doi.org/10.2166/aqua.2016.013
Michalak, I., Chojnacka, K., Dobrzański, Z., Górecki, H., Zielińska, A., Korczyński, M., Opaliński, S.: Effect of enriched with microelements macroalgae on egg quality parameters and mineral content of eggs, eggshell, blood, feathers and droppings. J. Anim. Physiol. Anim. Nutr. 95, 374–387 (2011). https://doi.org/10.1111/j.1439-0396.2010.01065
Michalak, I., Chojnacka, K., Korniewicz, D.: New feed supplement from macroalgae as the dietary source of microelements for pigs. Open Chem. 13, 1341–1352 (2015). https://doi.org/10.1515/chem-2015-0149
Witkowska, Z., Michalak, I., Korczyński, M., Szołtysik, M., Świniarska, M., Dobrzański, Z., Tuhy, Ł, Samoraj, M., Chojnacka, K.: Biofortification of milk and cheese with microelements by dietary feed bio-preparations. J. Food Sci. Technol. 52(10), 6484–6492 (2015). https://doi.org/10.1007/s13197-014-1696-9
Basinska, K., Marycz, K., Śmieszek, A., Nicpoń, J.: The production and distribution of IL-6 and TNF-α in subcutaneous adipose tissue and their correlation with serum concentrations in Welsh ponies with equine metabolic syndrome. J. Vet. Sci. 16(1), 113–120 (2015). https://doi.org/10.4142/jvs.2015.16.1.113
Chameroy, K.A.: (2010) Diagnosis and management of horses with Equine Metabolic Syndrome (EMS). PhD Dissertation, University of Tennessee, Knoxville, USA. http://trace.tennessee.edu/utk_graddiss/871. Accessed 28 Sept 2016
Frank, N., Geor, R.J., Bailey, S.R., Durham, A.E., Johnson, P.J.: Equine metabolic syndrome. J. Vet. Intern. Med. 24, 467–475 (2010). https://doi.org/10.1111/j.1939-1676.2010.0503.x
Marycz, K., Moll, E., Grzesiak, J.: Influence of functional nutrients on insulin resistance in horses with equine metabolic syndrome. Pak. Vet. J. 34(2), 189–192 (2014)
McGowan, C.M., Dugdale, A.H., Pinchbeck, G.L., Argo, C.M.G.: Dietary restriction in combination with a nutraceutical supplement for the management of equine metabolic syndrome in horses. Veter. J. 196, 153–159 (2013). https://doi.org/10.1016/j.tvjl.2012.10.007
Longland, A.C., Byrd, B.M.: Pasture nonstructural carbohydrates and equine laminitis. J. Nutr. 136, 2099S–2102S (2006)
Argo, C.M.G., Dugdale, A.H.A., McGowan, C.M.: Considerations for the use of restricted, soaked grass hay diets to promote weight loss in the management of equine metabolic syndrome and obesity. Veter. J. 206(2), 170–177 (2015). https://doi.org/10.1016/j.tvjl.2015.07.027
Aksu, Z.: Equilibrium and kinetic modelling of Cd(II) biosorption by C. vulgaris in batch system: effect of temperature. Sep. Purif. Technol. 21, 285–294 (2001)
Michalak, I., Chojnacka, K.: The new application of biosorption properties of Enteromorpha prolifera. Appl. Biochem. Biotechnol. 160, 1540–1556 (2010). https://doi.org/10.1007/s12010-009-8635-7
Ni, Y., Chen, S., Kokot, S.: Spectrophotometric determination of metal ions in electroplating solutions in the presence of EDTA with the aid of multivariate calibration and artificial neural networks. Anal. Chim. Acta 463, 305–316 (2002). https://doi.org/10.1016/S0003-2670(02)00437-3
Langmuir, I.: The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 40, 1361–1403 (1918)
McKay, G., Blair, H.S., Gardner, J.R.: The adsorption of dyes onto chitin in fixed bed columns and batch adsorbers. J. Appl. Pol. Sci. 29, 1499–1514 (1984)
Michalak, I., Chojnacka, K.: Edible macroalga Ulva prolifera as microelemental feed supplement for livestock: the fundamental assumptions of the production method. World J. Microbiol. Biotechnol. 25, 997–1005 (2009). https://doi.org/10.1007/s11274-009-9976-7
Kaliński, K., Marycz, K., Czogała, J., Serwa, E., Janeczek, W.: An application of scanning electron microscopy combined with roentgen microanalysis (SEM-EDS) in canine urolithiasis. J. Electron. Microsc. (Tokyo) 61(1), 47–55 (2012)
Michalak, I., Chojnacka, K., Marycz, K.: Using ICP-OES and SEM-EDX in biosorption studies. Mikrochim. Acta 172(1–2), 65–74 (2011). https://doi.org/10.1007/s00604-010-0468-0
Witek-Krowiak, A., Podstawczyk, D., Chojnacka, K., Dawiec, A., Marycz, K.: Modelling and optimization of chromium III biosorption on soybean meal. Cent. Eur. J. Chem. 11(9), 1505–1517 (2013). https://doi.org/10.2478/s11532-013-0274-8
Yahaya, Y.A., Don, M.M.: Pycnoporus sanguineus as potential biosorbent for heavy metal removal from aqueous solution: a review. J. Phys. Sci. 25(1), 1–32 (2014)
Yun, Y.S., Park, D., Park, J.M., Volesky, B.: Biosorption of trivalent chromium on the brown seaweed biomass. Environ. Sci. Technol. 35, 4353–4358 (2001). https://doi.org/10.1021/es010866k
Samoraj, M., Tuhy, Ł, Chojnacka, K.: New bench scale plant for biosorption. Proceedings of TINOS 2015 3rd International Conference on Sustainable Solid Waste Management. TINOS Island, Greece, 24.07.2015 (2018). http://uest.ntua.gr/tinos2015/proceedings/pdfs/Samoraj.pdf. Accessed 10 Apr 2018
Acknowledgements
This project is financed in the framework of grant entitled—“The effect of bioactive algae enriched by biosorption on the certain minerals such as Cr(III), Mg(II) and Mn(II) on the status of glucose in the course of metabolic syndrome horses. Evaluation in vitro and in vivo” (2015/18/E/NZ9/00607) attributed by The National Science Centre in Poland.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Michalak, I., Godlewska, K. & Marycz, K. Biomass Enriched with Minerals via Biosorption Process as a Potential Ingredient of Horse Feed. Waste Biomass Valor 10, 3403–3418 (2019). https://doi.org/10.1007/s12649-018-0351-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-018-0351-5









