Abstract
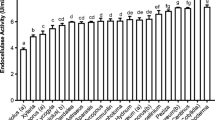
The efficient use of lignocellulosic materials for pharmaceutical and chemical production relies on the hydrolysis of their components into their building blocks (e.g. hexoses and pentoses), which can be converted later into chemicals of interest. This study aimed to isolate cellulase- and xylanase-producing bacteria for the bioconversion of lignocellulosic materials into their respective monomeric sugars. Bacterial isolates were screened using CMC- and Xylan–Trypan blue agar, and then cellulase and xylanase activities were evaluated by the 3,5-dinitro-salicylic acid (DNS) method. Furthermore, bacterial ability to saccharify wheat straw was tested. Ten bacterial isolates were found to have the ability to saccharify wheat straw, and to produce cellulase and xylanase enzymes simultaneously. The bacterial isolates were identified at molecular level using 16S rRNA gene sequencing and phylogenetic analysis. Bacterial isolates were identified as Cellulomonas sp. CX4, Cellulomonas sp. CX5, Bacillus sp. CX6, Bacillus sp. CX10, Paenibacillus illinoisensis CX11, Paenibacillus sp. CX14, Bacillus cereus CX15, and Bacillus sp. CX16, Paenibacillus barcinonensis CX17 and Cellulomonas sp. CX20. Among all the isolates, Bacillus sp. CX6 showed the highest ability to produce total reducing sugar (6.03 and 6.16 mg/ml), while the lowest ability to saccharify wheat straw was found with Cellulomonas sp. CX5 (2.01 and 2.12 mg/ml). This study presents cellulase- and xylanase- producing bacterial isolates for their potential to saccharify lignocellulosic materials for possible use in the production of pharmaceuticals and chemicals.




Similar content being viewed by others
References
Summerton, L., Sneddon, H.F., Jones, L.C., Clark, J.H.: Green and Sustainable Medicinal Chemistry: Methods, Tools and Strategies for the 21st Century Pharmaceutical Industry. Royal Society of Chemistry, CPI group Ltd, Croydon, UK (2016)
Cherubini, F., Strømman, A.H.: Chemicals from lignocellulosic biomass: opportunities, perspectives, and potential of biorefinery systems. Biofuels Bioprod. Biorefin. 5(5), 548–561 (2011).
Wongwilaiwalin, S., Rattanachomsri, U., Laothanachareon, T., Eurwilaichitr, L., Igarashi, Y., Champreda, V.: Analysis of a thermophilic lignocellulose degrading microbial consortium and multi-species lignocellulolytic enzyme system. Enzym. Microb. Technol. 47(6), 283–290 (2010)
Menon, V., Rao, M.: Trends in bioconversion of lignocellulose: biofuels, platform chemicals & biorefinery concept. Prog. Energy Combust. Sci. 38(4), 522–550 (2012)
Chesson, A., Forsberg, C.: Polysaccharide degradation by rumen microorganisms. In: The Rumen Microbial Ecosystem, pp. 329–381. Springer, New York (1997)
Maki, M.L., Broere, M., Leung, K.T., Qin, W.: Characterization of some efficient cellulase producing bacteria isolated from paper mill sludges and organic fertilizers. Int. J. Biochem. Mol. Biol. 2(2), 146–154 (2011)
Chang, V.S., Holtzapple, M.T.: Fundamental factors affecting biomass enzymatic reactivity. In: Twenty-First Symposium on Biotechnology for Fuels and Chemicals. pp. 5–37. Springer, New York (2000)
Ehsanipour, M., Suko, A.V., Bura, R.: Fermentation of lignocellulosic sugars to acetic acid by Moorella thermoacetica. J. Ind. Microbiol. Biotechnol. 43(6), 807–816 (2016). doi:10.1007/s10295-016-1756-4
Akhtar, J., Idris, A., Aziz, R.A.: Recent advances in production of succinic acid from lignocellulosic biomass. Appl. Microbiol. Biotechnol. 98(3), 987–1000 (2014)
Corma, A., Iborra, S., Velty, A.: Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 107(6), 2411–2502 (2007)
Huang, X., Chen, M., Lu, X., Li, Y., Li, X., Li, J.-J.: Direct production of itaconic acid from liquefied corn starch by genetically engineered Aspergillus terreus. Microb. Cell Fact. 13(1), 1 (2014)
Kautola, H.: Itaconic acid production from xylose in repeated-batch and continuous bioreactors. Appl. Microbiol. Biotechnol. 33(1), 7–11 (1990)
Isikgor, F.H., Becer, C.R.: Lignocellulosic biomass: a sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 6(25), 4497–4559 (2015).
Kurakake, M., Ide, N., Komaki, T.: Biological pretreatment with two bacterial strains for enzymatic hydrolysis of office paper. Curr. Microbiol. 54(6), 424–428 (2007)
Salvachúa, D., Prieto, A., López-Abelairas, M., Lu-Chau, T., Martínez, Á.T., Martínez, M.J.: Fungal pretreatment: an alternative in second-generation ethanol from wheat straw. Bioresour. Technol. 102(16), 7500–7506 (2011)
Venkateswar Rao, L., Goli, J.K., Gentela, J., Koti, S.: Bioconversion of lignocellulosic biomass to xylitol: an overview. Bioresour. Technol. 213, 299–310 (2016). doi:10.1016/j.biortech.2016.04.092
Chaturvedi, V., Verma, P.: An overview of key pretreatment processes employed for bioconversion of lignocellulosic biomass into biofuels and value added products. 3. Biotech. 3(5), 415–431 (2013)
Maurya, D.P., Singla, A., Negi, S.: An overview of key pretreatment processes for biological conversion of lignocellulosic biomass to bioethanol. 3 Biotech. 5(5), 597–609 (2015)
Gibson, D.M., King, B.C., Hayes, M.L., Bergstrom, G.C.: Plant pathogens as a source of diverse enzymes for lignocellulose digestion. Curr. Opin. Microbiol. 14(3), 264–270 (2011)
Dashtban, M., Schraft, H., Qin, W.: Fungal bioconversion of lignocellulosic residues; opportunities & perspectives. Int. J. Biol. Sci. 5(6), 578 (2009)
Gincy, M., Sukumaran, R.K., Singhania, R.R., Pandey, A.: Progress in research on fungal cellulases for lignocellulose degradation. J. Sci. Ind. Res. 67, 898–907 (2008).
Shallom, D., Shoham, Y.: Microbial hemicellulases. Curr. Opin. Microbiol. 6(3), 219–228 (2003)
Yoshida, M., Liu, Y., Uchida, S., Kawarada, K., Ukagami, Y., Ichinose, H., Kaneko, S., Fukuda, K.: Effects of cellulose crystallinity, hemicellulose, and lignin on the enzymatic hydrolysis of Miscanthus sinensis to monosaccharides. Biosci. Biotechnol. Biochem. 72(3), 805–810 (2008)
Hu, J., Arantes, V., Saddler, J.N.: The enhancement of enzymatic hydrolysis of lignocellulosic substrates by the addition of accessory enzymes such as xylanase: is it an additive or synergistic effect? Biotechnol. Biofuels. 4(1), 1 (2011).
Kumar, R., Wyman, C.E.: Effect of xylanase supplementation of cellulase on digestion of corn stover solids prepared by leading pretreatment technologies. Bioresour. Technol. 100(18), 4203–4213 (2009)
Qing, Q., Wyman, C.E.: Supplementation with xylanase and β-xylosidase to reduce xylo-oligomer and xylan inhibition of enzymatic hydrolysis of cellulose and pretreated corn stover. Biotechnol. Biofuels 4(1), 1 (2011).
Gusakov, A.V.: Alternatives to Trichoderma reesei in biofuel production. Trends Biotechnol. 29(9), 419–425 (2011)
Miller, G.L.: Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31(3), 426–428 (1959)
Ghose, T.: Measurement of cellulase activities. Pure Appl. Chem. 59(2), 257–268 (1987)
Robson, L.M., Chambliss, G.H.: Cellulases of bacterial origin. Enzyme Microb. Technol. 11(10), 626–644 (1989). doi:10.1016/0141-0229(89)90001-X
Patagundi, B.I., Kaliwal, B.: Isolation and characterization of cellulase producing bacteria from soil. Int. J. Curr. Microbiol. Appl. Sci. 3(5), 59–69 (2014)
Subramaniyan, S., Prema, P.: Cellulase-free xylanases from Bacillus and other microorganisms. FEMS Microbiol. Lett. 183(1), 1–7 (2000)
Asha, B.M., Revathi, M., Yadav, A., Sakthivel, N.: Purification and characterization of a thermophilic cellulase from a novel cellulolytic strain, Paenibacillus barcinonensis. J. Microbiol. Biotechnol. 22(11), 1501–1509 (2012).
Ladeira, S.A., Cruz, E., Delatorre, A.B., Barbosa, J.B., Martins, M.L.L: Cellulase production by thermophilic Bacillus sp. SMIA-2 and its detergent compatibility. Electron. J. Biotechnol. 18(2), 110–115 (2015). doi:10.1016/j.ejbt.2014.12.008
Subramaniyan, S., Prema, P.: Biotechnology of microbial xylanases: enzymology, molecular biology, and application. Crit. Rev. Biotechnol. 22(1), 33–64 (2002). doi:10.1080/07388550290789450
Gaur, R., Tiwari, S.: Isolation, production, purification and characterization of an organic-solvent-thermostable alkalophilic cellulase from Bacillus vallismortis RG-07. BMC Biotechnol. 15, 19 (2015). doi:10.1186/s12896-015-0129-9
Sari, S., Pangstuti, A., Susilowati, A., Purwoko, T., Mahajoeno, E., Hidayat, W., Mardhena, I., Panuntun, D., Kurniawati, D., Anitasari, R.: Cellulolytic and hemicellulolytic bacteria from the gut of Oryctes rhinoceros larvae. Biodiversitas 17(1), 78–83 (2016). doi:10.13057/biodiv/d170111
Sharma, A., Tewari, R., Soni, S.: Application of statistical approach for optimizing CMCase production by Bacillus tequilensis S28 strain via submerged fermentation using wheat bran as carbon source. Int. J. Biol. Biomol. Agric. Food Biotechnol. Eng. 9(1), 76–86 (2015).
Alshelmani, M.I., Loh, T.C., Foo, H.L., Lau, W.H., Sazili, A.Q.: Biodegradation of palm kernel cake by cellulolytic and hemicellulolytic bacterial cultures through solid state fermentation. Sci. World J. 2014, 729852 (2014). doi:10.1155/2014/729852
Abo-State, M.A., El-Sheikh, H.H., El-Temtamy, S.A., Hosny, M.: Isolation and identification of bacterial strains for saccharification of agriculture wastes for bioethanol production. Int. J. Adv. Res. Biol. Sci. 3(2), 170–180 (2016)
Akhtar, M.S., Saleem, M., Akhtar, M.W.: Saccharification of lignocellulosic materials by the cellulases of Bacillus subtilis. Int. J. Agr. Biol. 3, 199–202 (2001)
Sangkharak, K., Vangsirikul, P., Janthachat, S.: Isolation of novel cellulase from agricultural soil and application for ethanol production. Int. J. Adv. Biotechnol. Res. 2(2), 230–239 (2011)
Saxena, S., Bahadur, J., Varma, A.: Production and localisation of carboxymethylcellulase, xylanase and β-glucosidase from Cellulomonas and Micrococcus spp. Appl. Microbiol. Biotechnol. 34(5), 668–670 (1991)
Poulsen, O., Petersen, L.: Growth of Cellulomonas sp. ATCC 21399 on different polysaccharides as sole carbon source induction of extracellular enzymes. Appl. Microbiol. Biotechnol. 29(5), 480–484 (1988)
Rajoka, M.I., Malik, K.A.: Cellulase and hemicellulase production by Cellulomonas flavigena NIAB 441. Biotechnol. Lett. 6(9), 597–600 (1984)
Rajoka, M.I., Malik, K.A.: Cellulase production by Cellulomonas biazotea cultured in media containing different cellulosic substrates. Bioresour. Technol. 59(1), 21–27 (1997)
Sanchez, M.M., Fritze, D., Blanco, A., Sproer, C., Tindall, B.J., Schumann, P., Kroppenstedt, R.M., Diaz, P., Pastor, F.I.: Paenibacillus barcinonensis sp. nov., a xylanase-producing bacterium isolated from a rice field in the Ebro River delta. Int. J. Syst. Evol. Microbiol. 55(Pt 2), 935–939 (2005). doi:10.1099/ijs.0.63383-0
Pason, P., Kyu, K.L., Ratanakhanokchai, K.: Paenibacillus curdlanolyticus strain B-6 xylanolytic-cellulolytic enzyme system that degrades insoluble polysaccharides. Appl. Environ. Microbiol. 72(4), 2483–2490 (2006)
Sharma, M., Mehta, S., Kumar, A.: Purification and characterization of alkaline xylanase secreted from Paenibacillus macquariensis. Adv. Microbiol. 3, 32–41 (2013).
Rivas, R., García-Fraile, P., Mateos, P.F., Martínez-Molina, E., Velázquez, E.: Paenibacillus cellulosilyticus sp. nov., a cellulolytic and xylanolytic bacterium isolated from the bract phyllosphere of Phoenix dactylifera. Int. J. Syst. Evol. Microbiol. 56(12), 2777–2781 (2006)
Zhou, C.H., Xia, X., Lin, C.X., Tong, D.S., Beltramini, J.: Catalytic conversion of lignocellulosic biomass to fine chemicals and fuels. Chem. Soc. Rev. 40(11), 5588–5617 (2011). doi:10.1039/c1cs15124j
Ragauskas, A.J., Williams, C.K., Davison, B.H., Britovsek, G., Cairney, J., Eckert, C.A., Frederick, W.J., Hallett, J.P., Leak, D.J., Liotta, C.L.: The path forward for biofuels and biomaterials. Science. 311(5760), 484–489 (2006)
Mäki-Arvela, P., Anugwom, I., Virtanen, P., Sjöholm, R., Mikkola, J.P.: Dissolution of lignocellulosic materials and its constituents using ionic liquids—a review. Ind. Crops Prod. 32(3), 175–201 (2010). doi:10.1016/j.indcrop.2010.04.005
Sun, N., Rodriguez, H., Rahman, M., Rogers, R.D.: Where are ionic liquid strategies most suited in the pursuit of chemicals and energy from lignocellulosic biomass?. Chem. Commun. 47(5), 1405–1421 (2011). doi:10.1039/c0cc03990j. (Cambridge, England)
Barakat, A., de Vries, H., Rouau, X.: Dry fractionation process as an important step in current and future lignocellulose biorefineries: a review. Bioresour. Technol. 134, 362–373 (2013). doi:10.1016/j.biortech.2013.01.169
Kobayashi, H., Fukuoka, A.: Synthesis and utilisation of sugar compounds derived from lignocellulosic biomass. Green Chem. 15(7), 1740–1763 (2013). doi:10.1039/C3GC00060E
Acknowledgements
AAQA and OOB are grateful for the research support provided by North-West University. AAQA and TM are thankful to the University of South Africa (UNISA) for the valuable support for their research. OOB would like to thank the National Research Foundation, South Africa for grant (Ref: UID91990), which has supported research in her laboratory.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahmed, A.A.Q., Babalola, O.O. & McKay, T. Cellulase- and Xylanase-Producing Bacterial Isolates with the Ability to Saccharify Wheat Straw and Their Potential Use in the Production of Pharmaceuticals and Chemicals from Lignocellulosic Materials. Waste Biomass Valor 9, 765–775 (2018). https://doi.org/10.1007/s12649-017-9849-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-017-9849-5