Abstract
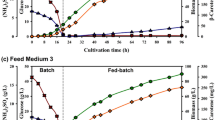
Due to the high price of animal feed additive, a number of studies have been carried out to find an alternative diet with comparable nutritional profile and cost competitiveness. The photosynthetic bacterium Rhodopseudomonas faecalis PA2 was recently proposed as a new carotenoid producer with relatively high biomass production but the mass production using a cheap substrate remains unclear. In this study, Rps. faecalis PA2 was cultivated in domestic wastewater. The optimum light intensity and agitation speed were 4000 lux and 150 rpm, respectively. Batch cultivation in a photo-bioreactor showed that specific growth rate was 1.61/day with the maximum biomass production of 33.9 g/L. Carotenoid yield, carotenoid production rate and carotenoid productivity were found to be 7.2 mg/g, 74.3 and 40.9 mg/L day, respectively. The nutritional profile of the freeze-dried biomass contained 64.8% protein and 10.6% lipid. The contents of essential amino acids accounted for approximately 72.6% of the whole protein content which is comparable with dietary amino acids required for penaeid shrimp. The amounts of unsaturated fatty acids were higher than saturated fatty acids consisting of polyunsaturated fatty acid including omega-3 (alpha-linolenic acid [18, n − 3]) and omega-6 fatty acids. Cultivation of this bacterium in domestic wastewater is considered a promising technology for microbial feed additive production with price competitiveness. Moreover, biochemical composition of Rps. faecalis grown in domestic wastewater is first reported in this study.





Similar content being viewed by others
References
Nasseri, A.T., Rasoul-Amini, S., Morowvat, M.H., Ghasemi, Y.: Single protein: production and process. Am. J. Food Technol. 6, 103–116 (2011)
Calozzi, P., Buccioni, A., Minieri, S., Pushparaj, B., Piccardi, R., Ena, A., Pintucci, C.: Production of bio-fuels (hydrogen and lipids) through a photofermentation process. Bioresour. Technol. 101, 3115–3120 (2010)
Kim, J.K., Lee, B.: Mass production of Rhodopseudomonas palustris as diet for aquaculture. Aquac. Eng. 23, 281–293 (2000)
Tian, Y., Machado, P.A., Fu, H., Hahm, T.S., Wei, C., Lo, Y.M.: Photosynthetic bioconversion of coenzyme Q10 using agrowaste generated from tobacco biorefinery for nonsmoking applications: a review. J. Food Drug Anal. 20, 173–178 (2012)
Wang, G., Grammel, H., Abou-Aisha, K., Sagesser, R., Ghosh, R.: High-level production of the industrial product lycopene by the photosynthetic bacterium Rhodospirillum rubrum. Appl. Environ. Microbiol. 78, 7205–7215 (2012)
Brown, A.C., McGraw, K.J., Clotfelter, E.D.: Dietary carotenoids increase yellow nonpigment coloration of female convict cichlids (Amantitlania nigrofasciata). Physiol. Biochem. Zool. 86, 312–322 (2013)
Takaichi, S., Maoka, T., Sasikala, C., Ramana, C.V., Shimada, K.: Genus specific unusual carotenoids in purple bacteria, Phaeospirillum and Roseospira: structures and biosyntheses. Curr. Microbiol. 63, 75–80 (2011)
Zhou, Q., Zhang, P., Zhang, G.: Biomass and carotenoid production in photosynthetic bacteria wastewater treatment: effect of light intensity. Bioresour. Technol. 171, 330–335 (2014)
Zhou, Q., Zhang, P., Zhang, G.: Biomass and pigments production in photosynthetic bacteria wastewater treatment: effects of light sources. Bioresour. Technol. 179, 505–509 (2015)
Loo, P.L., Vikineswary, S., Chong, V.C.: Nutritional value and production of three species of purple non-sulfur bacteria grown in palm oil mill effluent and their application in rotifer culture. Aquac. Nutr. 19, 895–907 (2013)
Saejung, C., Thammaratana, T.: Biomass recovery during municipal wastewater treatment using photosynthetic bacteria and prospect of production of single cell protein for feedstuff. Environ. Technol. 37, 3055–3061 (2016)
Chiu, K., Liu, W.: Dietary administration of the extract of Rhodobacter sphaeroides WL-APD911 enhances the growth performance and innate immune responses of seawater red tilapia (Oreochromis mossambicus × Oreochromis niloticus). Aquaculture 418–419, 32–38 (2014)
Fang, L.C., Li, Y., Cheng, P., Deng, J., Jiang, L.L., Huang, H., Zheng, J.S., Wei, H.: Characterization of Rhodopseudomonas palustris strain 2C as a potential probiotic. APMIS 120, 743–749 (2012)
Loo, P.L., Chong, V.C., Vikineswary, S.: Rhodovulum sulfidophilum, a phototrophic bacterium, grown in palm oil mill effluent improves the larval survival of marble goby Oxyeleotris marmorata (Bleeker). Aquac. Res. 44, 495–507 (2013)
Wu, P., Li, J., Wang, Y., Tong, Q., Liu, W., Du, C., Li, N.: Improving the growth of Rubrivivax gelatinosus cultivated in sewage environment. Bioprocess Biosyst. Eng. 38, 79–84 (2015)
Zhang, D., Yang, H., Huang, Z., Zhang, W., Liu, S.J.: Rhodopseudomonas faecalis sp. nov., a phototrophic bacterium isolated from an anaerobic reactor that digests chicken faeces. Int. J. Syst. Evol. Microbiol. 52, 2055–2060 (2002)
Hong, H.Y., Liu, B., Ding, J., Nan, J., Xie, G., Zhao, L., Chen, M., Ren, N.: Enhanced photo-hydrogen production of Rhodopseudomonas faecalis RLD-53 by EDTA addition. Int. J. Hydrogen Energy. 37, 8277–8281 (2012)
Liu, B., Xie, G., Guo, W., Ding, J., Ren, N.Q.: Optimization of photo-hydrogen production by immobilized Rhodopseudomonas faecalis RLD-53. Nat. Resour. 2, 1–7 (2011)
Xie, G., Liu, B., Xing, D., Nan, J., Ding, J., Ren, H.Y., Guo, W., Ren, N.Q.: Photo- hydrogen production by Rhodopseudomonas faecalis RLD-53 immobilized on the surface of modified activated carbon fibers. RSC Adv. 2, 2225–2228 (2012)
Saejung, C., Apaiwong, P.: Enhancement of carotenoid production in the new carotenoid-producing photosynthetic bacterium Rhodopseudomonas faecalis PA2. Biotechnol. Bioprocess Eng. 20, 701–707 (2015)
Association of Official Analytical Chemists (AOAC): Offcial Methods of Analysis. 16th edn. AOAC Arlington, Virginia (1995)
Association of Offcial Analytical Chemists (AOAC): Official Methods of Analysis, 24th edn. AOAC Arlington, Virginia (2000)
Association of Offcial Analytical Chemists (AOAC): Official Methods of Analysis, 24th edn. AOAC Arlington, Virginia (2005)
Hirayama, O.: Lipids and lipoprotein complex in photosynthetic tissue: 4 lipid and pigments of photosynthetic bacteria. Agric. Biol. Chem. 32, 34–41 (1968)
Cheirsilp, B., Torpee, S.: Enhanced growth and lipid production of microalgae under mixotrophic culture condition: effect of light intensity, glucose concentration and fed-batch cultivation. Bioresour. Technol. 110, 510–516 (2012)
Couso, I., Vila, M., Vigara, J., Cordero, B.F., Vargas, M.A., Guez, H.R., Leo, R.: Synthesis of carotenoids and regulation of the carotenoid biosynthesis pathway in response to high light stress in the unicellular microalga Chlamydomonas reinhardtii. Eur. J. Phycol. 47, 223–232 (2012)
Hosseini Tafreshi, A., Shariati, M.: Dunaliella biotechnology: methods and applications. J. Appl. Microbiol. 107, 14–35 (2009)
Kuo, F.S., Chien, Y.H., Chen, C.J.: Effects of light sources on growth and carotenoid content of photosynthetic bacteria Rhodopseudomonas palustris. Bioresour. Technol. 113, 315–318 (2012)
Li, Q., Brendemuhl, J.H., Jeong, K.C., Badinga, L.: Effects of dietary omega-3 polyunsaturated fatty acids on growth and immune response of weanling pigs. J. Anim. Sci. Technol. 56, 7 (2014)
Tandori, J., Hideg, E., Nagy, L., Maroti, P., Vass, I.: Photoinhibition of carotenoidless reaction centers from Rhodobacter sphaeroides by visible light. Effects on protein structure and electron transport. Photosynth. Res. 70, 175–184 (2001)
Masuda, T., Polle, J.E.W., Melis, A.: Biosynthesis and distribution of chlorophyll among the photosystems during recovery of the green alga Dunaliella salina from irradiance stress. Plant Physiol. 128, 603–614 (2002)
Brotosudarmo, T.H.P., Limantara, L., Heriyantoa, H., Prihastyanti, M.N.U.: Adaptation of the photosynthetic unit of purple bacteria to changes of light illumination intensities. Procedia Chem. 14, 414–421 (2015)
Nunkaew, T., Kantachote, D., Nitoda, T., Kanzaki, H.: The use of rice straw broth as an appropriate medium to isolate purple nonsulfur bacteria from paddy fields. Electron. J. Biotechnol. (2012). doi:10.2225/vol15-issue6-fulltext-8
Sangkharak, K., Prasertsan, P.: Nutrient optimization for production of polyhydroxybutyrate from halotolerant photosynthetic bacteria cultivated under aerobic-dark condition. Electron. J. Biotechnol. (2008). doi:10.2225/vol11-issue3-fulltext-2
Liu, S., Zhang, G., Li, X., Wu, P., Zhang, J.: Enhancement of Rhodobacter sphaeroides growth and carotenoid production through biostimulation. J. Environ. Sci. 33, 21–28 (2015)
Mitra, D., Leeuwen, J.H., Lamsal, B.: Heterotrophic/mixotrophic cultivation of leaginous Chlorella vulgaris on industrial co-products. Algal Res. 1, 40–48 (2012)
Vrati, S.: Single cell protein production by photosynthetic bacteria grown on the clarified effluents of biogas plant. Appl. Microbiol. Biotechnol. 19, 199–202 (1984)
Ponsano, E.H.G., Lacava, P.M., Pinto, M.F.: Chemical composition of Rhodocyclus gelatinosus biomass produced in poultry slaughterhouse wastewater. Braz. Arch. Biol. Technol. 46, 143–147 (2003)
Kobayashi, M., Kobayashi, M.: Waste remediation and treatment using anoxygenic phototrophic bacteria. In: Blackenship, R.E., Madigan, M.T., Bauer, C.E. (eds.) Anoxygenic Photosynthetic Bacteria, pp. 1269–1282. Kluwer Academic Publishers, Amsterdams (1995)
Noparatnaraporn, N., Nagai, S.: Selection of Rhodobactor sphaeroides P47 as useful source of single cell protein. J. Gen. Appl. Microbiol. 32, 351–359 (1986)
Becker, E.W.: Micro-algae as a source of protein. Biotechnol. Adv. 25, 207–210 (2007)
Tacon, A.G.J.: Standard Methods for the Nutrition and Feeding of Farmed Fish and Shrimp. Argent Laboratories Press, Washington (1990)
Oura, E.: Biomass from carbohydrates. In: Dellweg, H. (ed.) Biotechnology, pp. 3–42. Verlag Chemie GmbH, Weinheim (1983)
Gao, Y., Li, D., Liu, Y.: Production of single cell protein from soy molasses using Candida tropicalis. Ann. Microbiol. 62, 1165–1172 (2012)
Zhao, G., Zhang, W., Zhang, G.: Production of single cell protein using waste capsicum powder produced during capsanthin extraction. Lett. Appl. Microbiol. 50, 187–191 (2010)
Ashokkumar, G.V., Sujatha, R.K., Thiruneelakandan, G., Rashmi, V.: Diversity of purple non sulfur bacteria (PNSB) from shrimp ponds in Nagai coastal region, South East coast of India. Microbiology 1, 59–61 (2015)
FAO: Amino Acid Content of Foods and Biological Data on Proteins. Vol. 24, FAO Nutritional Studies, Rome (1980)
Syadati, S.A., Mirzaei-Aghsaghali, A., Fathi, H., Davuodi, J.: Importance essential fatty acids (n-6 and n-3) in animal nutrition: I: Ruminant. Ann. Biol. Res. 3, 1161–1176 (2012)
Gonzalez-Esquerra, R., Leeson, S.: Alternatives for enrichment of eggs and chicken meat with omega-3 fatty acids. Can. J. Anim. Sci. 81, 295–305 (2001)
Acknowledgements
This work was supported by the Thailand Research Fund (TRF) and the Office of the Higher Education Commission (Grant No. MRG6080233) and Khon Kaen University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saejung, C., Ampornpat, W. Production and Nutritional Performance of Carotenoid-Producing Photosynthetic Bacterium Rhodopseudomonas faecalis PA2 Grown in Domestic Wastewater Intended for Animal Feed Production. Waste Biomass Valor 10, 299–310 (2019). https://doi.org/10.1007/s12649-017-0070-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-017-0070-3