Abstract
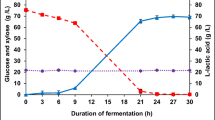
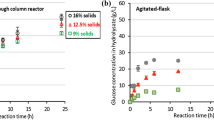
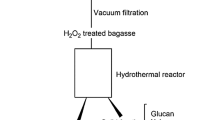
This work determined the optimal conditions to pretreat sucargane bagasse with HCl by using the liquid and the solid fractions resulting from the bagasse pretreament as substrate for fermentative hydrogen production by a mixed culture. A 23 full factorial central composite design (star configuration) helped to determine how temperature, time, and acid concentration affected the total monosaccharides (TM), total reducing sugars (TRS), and total inhibitors (TI) concentrations in the liquid fraction. Temperature, time, and acid concentration impacted the TRS and TM concentrations, but these variables did not influence the TI concentration significantly. The optimal pretreatment conditions were HCl at 7.36 % (v/v), 96.8 °C, and 441.6 min, which afforded the highest TRS concentration in the liquid hydrolysates. The liquid fraction obtained from the bagasse pretreated with acid under the optimal conditions (designated liq) was not suitable for H2 production by the mixed culture before treatment of the fraction with activated carbon. The solid residual bagasse (designated sol) alone afforded 6.0 mL of H2/g of bagasse. Liq treated with 10 % (m/v) activated carbon, to give liq + C, and sol added with the enzyme Celluclast® 10 U g−1, to afford sol + E, yielded 45.3 and 7.8 mL of H2/g of bagasse respectively, which amounted to 53.1 mL of H2/g of bagasse. The volumetric productivities—1450 and 1423 mL of H2 L−1 d−1 for liq + C and sol + E, respectively—are the highest ever reported in the literature for H2 production from sugarcane bagasse by a microbial consortium.



Similar content being viewed by others
References
Instituto Brasileiro de Geografia e Estatística - IBGE. Anuário Estatístico do Brasil, v.73, ISSN 0100-1299 (2013)
Hofsetz, K., Silva, M.A.: Brazilian sugarcane bagasse: energy and non-energyconsumption. Biomass Bioenergy 46, 564–573 (2012)
Pattra, S., Sangyoka, S., Boonmee, M., Reungsang, A.: Bio-hydrogen production from the fermentation of sugarcane bagasse hydrolysate by Clostridium butyricum. Int. J. Hydrog. Energy 33, 5256–5265 (2008)
Chairattanamanokorn, P., Penthamkeerati, P., Reungsang, A., Lo, Y.-C., Lu, W.B., Chang, J.S.: Production of biohydrogen from hydrolyzed bagasse with thermally preheated sludge. Int. J. Hydrog. Energy 34, 7612–7617 (2009)
Cheng, C.L., Chang, J.S.: Hydrolysis of lignocellulosic feedstock by novel cellulases originating from Pseudomonas sp. CL3 for fermentative hydrogen production. Bioresour. Technol. 102, 8628–8634 (2011)
Fangkum, A., Reungsang, A.: Biohydrogen production from sugarcane bagasse hydrolysate by elephant dung: effects of initial pH and substrate concentration. Int. J. Hydrog. Energy 36, 8687–8696 (2011)
Lai, Z., Zhu, M., Yang, X., Wang, J., Li, S.: Optimization of key factors affecting hydrogen production from sugarcane bagasse by a thermophilic anaerobic pure culture. Biotechnol. Biofuels 7, 119–130 (2014)
Ntaikou, I., Antonopoulou, G., Lyberatos, G.: Biohydrogen production from biomass and wastes via dark fermentation: a review. Waste Biomass Valor. 1, 21–39 (2010)
Rezende, C.A., de Lima, M.A., Maziero, P., de Azevedo, E.R., Garcia, W., Polikarpov, I.: Chemical and morphological characterization of sugarcane bagasse submitted to a delignification process for enhanced enzymatic digestibility. Biotechnol. Biofuels 4, 1–18 (2011)
Alvira, P., Tomás-Pejó, E., Ballesteros, M., Negro, M.J.: Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour. Technol. 101, 4851–4861 (2010)
Monlau, F., Barakat, A., Trably, E., Dumas, C., Steyer, J.-P., Carrere, H.: Lignocellulosic materials into biohydrogen and biomethane: impact of structural features and pretreatment. Crit. Rev. Environ. Sci. Technol. 43, 260–322 (2013)
Saripan, A.F., Reungsang, A.: Biohydrogen production by Thermoanaerobacterium thermosaccharolyticum KKU-ED1: culture conditions optimization using xylan as the substrate. Int. J. Hydrog. Energy 38, 6167–6173 (2013)
Lavarack, B.P., Griffin, G.J., Rodman, D.: The acid hydrolysis of sugarcane bagasse hemicellulose to produce xylose, arabinose, glucose and other products. Biomass Bioenergy 23, 367–380 (2002)
Jonsson, L., Alriksson, B.: Nilvebrant N–O bioconversion of lignocellulose: inhibitors and detoxification. Biotechnol. Biofuels 6, 16–22 (2013)
Valdez-Vazquez, I., Poggi-Varaldo, H.M.: Hydrogen production by fermentative consortia. Renew. Sustain. Energy Rev. 13, 1000–1013 (2009)
Derringer, G., Suich, R.: Simultaneous optimization of several response variables. J. Qual. Technol. 12, 214–219 (1980)
Gonzalez-Gil, G., Kleerebezem, R., Lettinga, G.: Assessment of metabolic properties and kinetic parameters of methanogenic sludge by on-line methane production rate measurements. Appl. Microbiol. Biotechnol. 58, 248–254 (2002)
Buitrón, G., Carvajal, C.: Biohydrogen production from Tequila vinasses in an anaerobic sequencing batch reactor: effect of initial substrate concentration, temperature and hydraulic retention time. Bioresour. Technol. 101, 9071–9077 (2010)
Hargraeves, P.I., Barcelos, C.A., Costa, A.C.A., Pereira Jr, N.: Production of ethanol 3G from Kappaphycus alvarezii: evaluation of different process strategies. Bioresour. Technol. 134, 257–263 (2013)
Ghose, T.K.: Measurement of cellulase activities. Pure Appl. Chem. 59, 257–268 (1987)
García-Morales, J.L., Nebot, E., Romero, L.I., Sales, D.: Comparison between acidogenic and methanogenic inhibition caused by liner alkylbenzzene-sulfonate (LAS). Chem. Biochem. Eng. Q. 15, 13–19 (2001)
Sun, J.: Isolation and characterization of cellulose from sugarcane bagasse. Polym. Degrad Stabil. 84, 331–339 (2004)
TAPPI. TAPPI Standard. Method T19 om-54. TAPPI test methods (1991)
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D. Determination of structural carbohydrates and lignin in biomass. Technical Report. NREL/TP-510-42618 (2012)
APHA, AWWA, WEF. Standard methods for the examination of water and wastewater. 19th. edn. American Public Health Association. Washington, DC (1995)
Miller, G.L.: Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal. Chem. 30, 785–793 (1959)
Sá, L.R.V., de Oliveira, M.A.L., Cammarota, M.C., Matos, A., Ferreira-Leitão, V.S.: Simultaneous analysis of carboydrates and volatile fatty acids by HPLC for monitoring fermentative biohydrogen production. Int. J. Hydrog. Energy 36, 7543–7549 (2011)
Monlau, F., Barakat, A., Trably, E., Dumas, C., Steyer, J.P., Carrère, H.: Lignocellulosic materials into biohydrogen and biomethane: impact of structural features and pretreatment. Crit. Rev. Environ. Sci. Technol. 43, 260–322 (2013)
Klinke, H.B., Thomsen, A.B., Ahring, B.K.: Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl. Microbiol. Biotechnol. 66, 10–26 (2004)
Hendriks, A.T.W.M., Zeeman, G.: Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 100, 10–18 (2009)
Rai, P.K., Singh, S.P., Asthana, R.K., Singh, S.: Biohydrogen production from sugarcane bagasse by integrating dark- and photo-fermentation. Bioresour. Technol. 152, 140–146 (2014)
Martin, C., Klinke, H., Thomsen, A.B.: Wet oxidation as a pretreatment method for enhancing the enzymatic convertibility of sugarcane bagasse. Enzyme Microbial. Technol. 40, 426–432 (2007)
Visser, E.M., Leal, T.F., Almeida, M.N., Guimarães, V.M.: Increased enzymatic hydrolysis of sugarcane bagasse from enzyme recycling. Biotechnol. Biofuels 8, 5 (2015)
Canilha, L., Carvalho, W., Felipe, M.G.A., Silva, J.B.A.: Xylitol production from wheat straw hemicellulosic hydrolysate: hydrolysate detoxification and carbon source used for inoculum preparation. Brazilian J. Microbiol. 39, 333–336 (2008)
Quermeneur, M., Hamelin, J., Barakat, A., Steyer, J.P., Carrere, H., Trably, E.: Inhibition of fermentative hydrogen production by lignocellulose-derived compounds in mixed cultures. Int. J. Hydrog. Energy 37, 3150–3159 (2012)
Wang, Y., Zhao, Q.B., Yang, M., Yu, H.Q., Harad, H., Li, Y.Y.: Biohydrogen production with mixed anaerobic cultures in the presence of high-concentration acetate. Int. J. Hydrog. Energy 33, 1164–1171 (2008)
Hernández-Salas, J.M., Villa-Ramírez, M.S., Veloz-Rendón, J.S., Rivera-Hernández, K.N., González-César, R.A., Plascencia-Espinosa, M.A., Trejo-Estrada, S.R.: Comparative hydrolysis and fermentation of sugarcane and agave bagasse. Bioresour. Technol. 100, 1238–1245 (2009)
Matsumoto, M., Nishimura, Y.: Hydrogen production by fermentation using acetic acid and lactic acid. J. Biosci. Bioeng. 103, 236–241 (2007)
Monlau, F., Sambusiti, C., Barakat, A., Quéméneur, M., Trably, E., Steyer, J.-P., Carrère, H.: Do furanic and phenolic compounds of lignocellulosic and algae biomass hydrolyzate inhibit anaerobic mixed cultures? A comprehensive review. Biotechnol. Adv. 32, 934–951 (2014)
Acknowledgments
The authors wish to thank ‘Programa de Aperfeiçoamento de Pessoal de Nível Superior’ (CAPES) as well as “Fundação de Amaro à Pesquisa do Estado de São Paulo” (FAPESP) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lorencini, P., Siqueira, M.R., Maniglia, B.C. et al. Biohydrogen Production from Liquid and Solid Fractions of Sugarcane Bagasse After Optimized Pretreatment with Hydrochloric Acid. Waste Biomass Valor 7, 1017–1029 (2016). https://doi.org/10.1007/s12649-016-9494-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-016-9494-4