Abstract
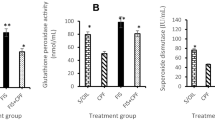
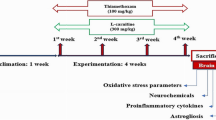
The current study aimed to investigate the role of fucoidan in the oxidative and apoptotic effects of sulfoxaflor, a neonicotinoid sulfoximine insecticide, in the brain of Swiss albino mice (Mus musculus). Sulfoxaflor and fucoidan were administered to mice at doses of 15 mg/kg/day (1/50 oral LD50) and 50 mg/kg/day, respectively, by oral gavage for 24 h or 7 days. The tGSH, TBARS and protein levels, and GPx, GR, and GST enzyme activities were determined by spectrophotometric methods. Caspase-3 gene expression level was determined by RT-PCR. Data analysis showed that brains of sulfoxaflor-treated mice exhibited higher TBARS levels; GPx, GR, and GST enzyme activities; and caspase-3 expression levels, as well as lower levels of tGSH. Co-administration of fucoidan and sulfoxaflor reduced the TBARS levels, increased tGSH levels, and increased GPx, GR, and GST enzyme activities. Fucoidan also decreased the sulfoxaflor-induced up-regulation of caspase-3 mRNA expression. Results of the present study showed that sulfoxaflor caused oxidative stress by inducing lipid peroxidation and altering GSH-dependent antioxidants in the brain of mice. In addition, sulfoxaflor may trigger apoptotic cell death shown by the up-regulation of caspase-3. Fucoidan treatment modulated all the aforementioned alterations in the brain of mice. It was concluded that fucoidan might have antioxidant effects that support the GSH-dependent antioxidant system and can play a modulator role in oxidative stress and caspase-3 expression in the brain of sulfoxaflor treated-mice.



Similar content being viewed by others
Availability of Data and Material
The data presented in this study are available on request from the corresponding author.
References
Abdel-Daim MM, Abdeen A, Jalouli M, Abdelkader A, Megahed A, Alkahtane A, Almeer R, Alhoshani NM, Al-Johani NS, Alkahtani S, Aleya L (2021) Fucoidan supplementation modulates hepato-renal oxidative stress and DNA damage induced by aflatoxin B1 intoxication in rats. Sci Total Environ 768:144781
Abdel-Daim MM, Dawood MAO, Aleya L, Alkahtani S (2020a) Effects of fucoidan on the hematic indicators and antioxidative responses of Nile tilapia (Oreochromis niloticus) fed diets contaminated with aflatoxin B1. Environ Sci Pollut Res Int 27(11):12579–12586
Abdel-Daim MM, Abushouk AI, Bahbah EI, Bungău SG, Alyousif MS, Aleya L, Alkahtani S (2020b) Fucoidan protects against subacute diazinon-induced oxidative damage in cardiac, hepatic, and renal tissues. Environ Sci Pollut Res Int 27(11):11554–11564
Abdollahi M, Ranjbar A, Shadnia S, Nikfar S, Rezaie A (2004) Pesticides and oxidative stress: a review. Med Sci Monit 10(6):RA141–147
Abou-Donia MB, Goldstein LB, Bullman S, Tu T, Khan WA, Dechkovskaia AM, Abdel-Rahman AA (2008) Imidacloprid induces neurobehavioral deficits and increases expression of glial fibrillary acidic protein in the motor cortex and hippocampus in offspring rats following in utero exposure. J Toxicol Environmen Health Part A 71(2):119–130
Ahmadi FA, Linseman DA, Grammatopoulos TN, Jones SM, Bouchard RJ, Freed CR, Heidenreich KA, Zawada WM (2003) The pesticide rotenone induces caspase-3-mediated apoptosis in ventral mesencephalic dopaminergic neurons. J Neurochem 87(4):914–921
Alam RT, Imam TS, Abo-Elmaaty AMA, Arisha AH (2019) Amelioration of fenitrothion induced oxidative DNA damage and inactivation of caspase-3 in the brain and spleen tissues of male rats by N-acetylcysteine. Life Sci 231:116534
Aleissa MS, Alkahtani S, Abd Eldaim MA, Ahmed AM, Bungău SG, Almutairi B, Bin-Jumah M, AlKahtane AA, Alyousif MS, Abdel-Daim MM (2020) Fucoidan ameliorates oxidative stress, inflammation, DNA damage, and hepatorenal injuries in diabetic rats intoxicated with aflatoxin B1. Oxid Med Cell Longev 2020:9316751
AlKahtane AA, Abushouk AI, Mohammed ET, ALNasser M, Alarifi S, Ali D, Alessia MS, Almeer RS, AlBasher G, Alkahtani S, Aleya L, Abdel-Daim MM (2020) Fucoidan alleviates microcystin-LR-induced hepatic, renal, and cardiac oxidative stress and inflammatory injuries in mice. Environ Sci Pollut Res Int 27(3):2935–2944
Allocati N, Masulli M, Di Ilio C, Federici L (2018) Glutathione transferases: substrates, inihibitors and pro-drugs in cancer and neurodegenerative diseases. Oncogenesis 7(1):8
Anadon A, Ares I, Martinez M, Martinez-Larranaga MR, Martinez M (2020) Neurotoxicity of neonicotinoids. In: Aschner M, Costa LG (eds) Advances in neurotoxicology. Elsevier Inc., USA, pp 167–207
Anderson ME (1985) Determination of glutathione and glutathione disulfide in biological samples. Method Enzymol 113:548–555
Anderson ME (1998) Glutathione: an overview of biosynthesis and modulation. Chem Biol Interact 111:1–14
Ando Y, Liang Y, Ishigaki S, Niwa J, Jiang Y, Kobayashi Y, Yamamoto M, Doyu M, Sobue G (2003) Caspase-1 and -3 mRNAs are differentially upregulated in motor neurons and glial cells in mutant SOD1 transgenic mouse spinal cord: a study using laser microdissection and real-time RT-PCR. Neurochem Res 28(6):839–846
Atashrazm F, Lowenthal RM, Woods GM, Holloway AF, Dickinson JL (2015) Fucoidan and cancer: a multifunctional molecule with anti-tumor potential. Mar Drugs 13(4):2327–2346
Aydin B (2011) Effects of thiacloprid, deltamethrin and their combination on oxidative stress in lymphoid organs, polymorphonuclear leukocytes and plasma of rats. Pestic Biochem Physiol 100:165–171
Bal R, Türk G, Tuzcu M, Yilmaz O, Kuloglu T, Gundogdu R, Gür S, Agca A, Ulas M, Cambay Z, Tuzcu Z, Gencoglu H, Guvenc M, Ozsahin AD, Kocaman N, Aslan A, Etem E (2012a) Assessment of imidacloprid toxicity on reproductive organ system of adult male rats. J Environ Sci Health B 47(5):434–444
Bal R, Türk G, Yılmaz Ö, Etem E, Kuloğlu T, Baydaş G, Naziroğlu M (2012b) Effects of clothianidin exposure on sperm quality, testicular apoptosis and fatty acid composition in developing male rats. Cell Biol Toxicol 28(3):187–200
Bauer S, Jin W, Zhang F, Linhardt RJ (2021) The application of seaweed polysaccharides and their derived products with potential for the treatment of alzheimer’s disease. Mar Drugs 19(2):89
Berteau O, Mulloy B (2003) Sulfated fucans, fresh perspectives: structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiol 13(6):29R-40R
Beutler E (1984) Red cell metabolism: a manual of biochemical methods. Grune and Starton, Orlando, USA.
Borsuah JF, Messer TL, Snow DD, Comfort SD, Mittelstet AR (2020) Literature review: global neonicotinoid ınsecticide occurrence in aquatic environments. Water 12(12):3388
Boshy M, Abdelhamidb F, Richab E, Ashshia A, Gaitha M, Qustya N (2017) Attenuation of CCl4 induced oxidative stress, immunosuppressive, hepatorenal damage by fucoidan in rats. J Clin Toxicol 7(348):2161–2495
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Brigelius-Flohé R, Maiorino M (2013) Glutathione peroxidases. Biochim Biophys Acta 1830(5):3289–3303
Brooks KJ, Wiescinski CM, Golden R (2008) MXDE-208: Acute oral toxicity study in CRL: CD1 (ICR) mice (up and down procedure). Midland, MI, USA, The Dow Chemical Company. DAS Report No. 081059. Submitted to WHO by Dow AgroSciences Europe, Abingdon, England
Carlberg INCER, Mannervik BENGT (1975) Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J Biol Chem 250(14):5475–5480
Chale-Dzul J, Pérez-Cabeza de Vaca R, Quintal-Novelo C, Olivera-Castillo L, Moo-Puc R (2020) Hepatoprotective effect of a fucoidan extract from Sargassum fluitans Borgesen against CCl4-induced toxicity in rats. Int J Biol Macromol 145:500–509
Cobley JN, Fiorello ML, Bailey DM (2018) 13 reasons why the brain is susceptible to oxidative stress. Redox Biol 15:490–503
Cumashi A, Ushakova NA, Preobrazhenskaya ME, D’Incecco A, Piccoli A, Totani L, Tinari N, Morozevich GE, Berman AE, Bilan MI, Usov AI, Ustyuzhanina NE, Grachev AA, Sanderson CJ, Kelly M, Rabinovich GA, Iacobelli S, Nifantiev NE (2007) A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiol 17(5):541–552
De Zwart LL, Meerman JH, Commandeur JN, Vermeulen NP (1999) Biomarkers of free radical damage applications in experimental animals and in humans. Free Radic Biol Med 26(1–2):202–226
Demeule M, Régina A, Jodoin J, Laplante A, Dagenais C, Berthelet F, Moghrabi A, Béliveau R (2002) Drug transport to the brain: key roles for the efflux pump P-glycoprotein in the blood-brain barrier. Vascul Pharmacol 38(6):339–348
Duzguner V, Erdogan S (2010) Acute oxidant and inflammatory effects of imidacloprid on the mammalian central nervous system and liver in rats. Pestic Biochem Physiol 97(1):13–18
El Okle OS, El Euony OI, Khafaga AF, Lebda MA (2018) Thiamethoxam induced hepatotoxicity and pro-carcinogenicity in rabbits via motivation of oxidative stress, inflammation, and anti-apoptotic pathway. Environ Sci Pollut Res Int 25(5):4678–4689
El-Gendy KS, Aly NM, Mahmoud FH, Kenawy A, El-Sebae AK (2010) The role of vitamin C as antioxidant in protection of oxidative stress induced by imidacloprid. Food Chem Toxicol 48(1):215–221
Elizondo-Gonzalez R, Cruz-Suarez LE, Ricque-Marie D, Mendoza-Gamboa E, Rodriguez-Padilla C, Trejo-Avila LM (2012) In vitro characterization of the antiviral activity of fucoidan from Cladosiphon okamuranus against Newcastle Disease Virus. Virol J 9:307
Ermakova S, Sokolova R, Um K-M, B-H, Isakov V, Zvyagintseva T (2011) Fucoidans from brown seaweeds Sargassum hornery, Eclonia cava, Costaria costata: structural characteristics and anticancer activity. Appl Biochem Biotechnol 164(6):841–850
Flohe L (2013) The fairytale of the GSSG/GSH redox potential. Biochim Biophy Acta 1830(5):3139–3142
Ford KA, Casida JE (2006) Chloropyridinyl neonicotinoid insecticides: diverse molecular substituents contribute to facile metabolism in mice. Chem Res Toxicol 19(7):944–951
Forman HJ, Zhang H, Kavanagh TJ (2018) Biosynthesis of glutathione and its regulation. In: Flohé L (ed) Glutathione, 1st edn, CRC Press. Boca Raton, pp 3–24
Garbarino VR, Orr ME, Rodriguez KA, Buffenstein R (2015) Mechanisms of oxidative stress resistance in the brain: lessons learned from hypoxia tolerant extremophilic vertebrates. Arch Biochem Biophys 576:8–16
Gupta RC (2012) Veterinary toxicology: basic and clinical principles. Academic press, Oxford, United Kingdom
Güney M, Oral B, Demirin H, Ozgüner M, Take G, Mungan T, Altuntas I (2007) Evaluation of caspase-dependent apoptosis during methyl parathion-induced endometrial damage in rats: Ameliorating effect of vitamins E and C. Environ Toxicol Pharmacol 23(2):221–227
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249(22):7130–9.
Hamidi S, Letourneur D, Aid-Launais R, Di Stefano A, Vainchenker W, Norol F, Le Visage C (2014) Fucoidan promotes early step of cardiac differentiation from human embryonic stem cells and long-term maintenance of beating areas. Tissue Eng Part A 20(7–8):1285–1294
Heeba GH, Morsy MA (2015) Fucoidan ameliorates steatohepatitis and insulin resistance by suppressing oxidative stress and inflammatory cytokines in experimental non-alcoholic fatty liver disease. Environ Toxicol Pharmacol 40:907–914
Hellou J, Ross NW, Moon TW (2012) Glutathione, glutathione S-transferase, and glutathione conjugates, complementary markers of oxidative stress in aquatic biota. Environ Sci Pollut Res Int 19(6):2007–2023
Hentati F, Tounsi L, Djomdi D, Pierre G, Delattre C, Ursu AV, Fendri I, Abdelkafi S, Michaud P (2020) Bioactive polysaccharides from seaweeds. Molecules 25(14):3152
Hong SW, Lee HS, Jung KH, Lee H, Hong SS (2012) Protective effect of fucoidan against acetaminophen-induced liver injury. Arch Pharm Res 35(6):1099–1105
Iraha A, Chinen H, Hokama A, Yonashiro T, Kinjo T, Kishimoto K, Nakamoto M, Hirata T, Kinjo N, Higa F, Tateyama M, Kinjo F, Fujita J (2013) Fucoidan enhances intestinal barrier function by upregulating the expression of claudin-1. World J Gastroenterol 19(33):5500–5507
Iwamoto K, Hiragun T, Takahagi S, Yanase Y, Morioke S, Mihara S, Kameyoshi Y, Hide M (2011) Fucoidan suppresses IgE production in peripheral blood mononuclear cells from patients with atopic dermatitis. Arch Dermatol Res 303(6):425–431
Jesberger JA, Richardson JS (1991) Oxygen free radicals and brain dysfunction. Int J Neurosci 57(1–2):1–17
Jeschke P, Nauen R, Schindler M, Elbert A (2011) Overview of the status and global strategy for neonicotinoids. J Agric Food Chem 59(7):2897–2908
Jhamandas JH, Wie MB, Harris K, MacTavish D, Kar S (2005) Fucoidan inhibits cellular and neurotoxic effects of beta-amyloid (A beta) in rat cholinergic basal forebrain neurons. Eur J Neurosci 21(10):2649–2659
Kang KS, Kim ID, Kwon RH, Lee JY, Kang JS, Ha BJ (2008) The effects of fucoidan extracts on CCl(4)-induced liver injury. Arch Pharm Res 31(5):622–627
Kapoor U, Srivastava MK, Srivastava LP (2011) Toxicological impact of technical imidacloprid on ovarian morphology, hormones and antioxidant enzymes in female rats. Food Chem Toxicol 49(12):3086–2089
Kurutas EB (2016) The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr J 15(1):71
Lanneau D, Brunet M, Frisan E, Solary E, Fontenay M, Garrido C (2008) Heat shock proteins: essential proteins for apoptosis regulation. J Cell Mol Med 12(3):743–761
Lean QY, Eri RD, Fitton JH, Patel RP, Gueven N (2015) Fucoidan extracts ameliorate acute colitis. PLoS One. 10(6):e0128453.
LeBaron MJ, Geter DR, Rasoulpour RJ, Gollapudi BB, Thomas J, Murray J, Kan HL, Wood AJ, Elcombe C, Vardy A, McEwan J, Terry C, Billington R (2013) An integrated approach for prospectively investigating a mode-of-action for rodent liver effects. Toxicol Appl Pharmacol 270(2):164–173
Lee BW, Oh SH, Chung JH, Moon CK, Lee BH (2004) N-nitroso metabolite of carbofuran induces apoptosis in CHL cells by cytochrome c-mediated activation of caspases. Toxicology 201(1–3):51–58
Li DY, Xu RY, Zhou WZ, Sheng XB, Yang AY, Cheng JL (2002) Effects of fucoidan extracted from brown seaweed on lipid peroxidation in mice. Acta Nutr Sinica 24:389–392
Li Y, Wei G, Chen J (2004) Glutathione: a review on biotechnological production. Appl Microbiol Biotechnol 66(3):233–242
Lim JD, Lee SR, Kim T, Jang SA, Kang SC, Koo HJ, Sohn E, Bak JP, Namkoong S, Kim HK, Song IS, Kim N, Sohn EH, Han J (2015) Fucoidan from Fucus vesiculosus protects against alcohol-induced liver damage by modulating inflammatory mediators in mice and HepG2 cells. Mar Drugs 13(2):1051–1067
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25(4):402–408
Lonare M, Kumar M, Raut S, Badgujar P, Doltade S, Telang A (2014) Evaluation of imidacloprid-induced neurotoxicity in male rats: A protective effect of curcumin. Neurochem Int 78:122–129
Lu Q, Sun Y, Ares I, Anadón A, Martínez M, Martínez-Larrañaga MR, Yuan Z, Wang X, Martínez MA (2019) Deltamethrin toxicity: a review of oxidative stress and metabolism. Environ Res 170:260–281
Lu SC (2013) Glutathione synthesis. Biochim Biophys Acta 1830(5):3143–3153
Luo D, Zhang Q, Wang H, Cui Y, Sun Z, Yang J, Zheng Y, Jia J, Yu F, Wang X, Wang X (2009) Fucoidan protects against dopaminergic neuron death in vivo and in vitro. Eur J Pharmacol 617(1–3):33–40
Ma XT, Sun XY, Yu K, Gui BS, Gui Q, Ouyang JM (2017) Effect of content of sulfate groups in seaweed polysaccharides on antioxidant activity and repair effect of subcellular organelles in Injured HK-2 Cells. Oxid Med Cell Longev 2017:2542950
Mahajan L, Verma PK, Raina R, Pankaj NK, Sood S, Singh M (2018) Alteration in thiols homeostasis, protein and lipid peroxidation in renal tissue following subacute oral exposure of imidacloprid and arsenic in Wistar rats. Toxicol Rep 5:1114–1119
Meenakshi S, Umayaparvathi S, Saravanan R, Manivasagam T, Balasubramanian T (2016) Neuroprotective effect of fucoidan from Turbinaria decurrens in MPTP intoxicated Parkinsonic mice. Int J Biol Macromol 86:425–433
Meenakshi S, Umayaparvathi S, Saravanan R, Manivasagam T, Balasubramanian T (2014) Hepatoprotective effect of fucoidan isolated from the seaweed Turbinaria decurrens in ethanol intoxicated rats. Int J Biol Macromol 67:367–372
Murakami K, Aoki H, Nakamura S, Nakamura S, Takikawa M, Hanzawa M, Kishimoto S, Hattori H, Tanaka Y, Kiyosawa T, Sato Y, Ishihara M (2010) Hydrogel blends of chitin/chitosan, fucoidan and alginate as healing-impaired wound dressings. Biomaterials 31(1):83–90
Nakamura S, Nambu M, Ishizuka T, Hattori H, Kanatani Y, Takase B, Kishimoto S, Amano Y, Aoki H, Kiyosawa T, Ishihara M, Maehara T (2008) Effect of controlled release of fibroblast growth factor-2 from chitosan/fucoidan micro complex-hydrogel on in vitro and in vivo vascularization. J Biomed Mater Res A 85(3):619–627
Nakazato K, Takada H, Iha M, Nagamine T (2010) Attenuation of N-nitrosodiethylamine-induced liver fibrosis by high-molecular-weight fucoidan derived from Cladosiphon okamuranus. J Gastroenterol Hepatol 25(10):1692–1701
Nguyen VT, Ko SC, Oh GW, Heo SY, Jeon YJ, Park WS, Choi IW, Choi SW, Jung WK (2016) Anti-inflammatory effects of sodium alginate/gelatine porous scaffolds merged with fucoidan in murine microglial BV2 cells. Int J Biol Macromol 93(Pt B):1620–1632
OECD (2001) Guideline for Testing of Chemicals. Acute Oral Toxicity, Acute Toxic Class Method
Ozdemir HH, Kara M, Yumrutas O, Uckardes F, Eraslan E, Demir CF, Bal R (2014) Determination of the effects on learning and memory performance and related gene expressions of clothianidin in rat models. Cogn Neurodyn 8(5):411–416
Özdemir S, Altun S, Arslan H (2018) Imidacloprid exposure cause the histopathological changes, activation of TNF-α, iNOS, 8-OHdG biomarkers, and alteration of caspase 3, iNOS, CYP1A.; MT1 gene expression levels in common carp (Cyprinus carpio L.). Toxicol Rep 5:125–133
Ponnan A, Kulanthaiyesu A, Marudhamuthu M, Palanisamy K, Kadarkarai M (2020) Protective effects of fucoidan against 4-nitroquinolin-1-oxide provoked genetic damage in mouse bone marrow cells. Environ Sci Pollut Res Int 27(25):31760–31766
Prasad S, Gupta SC, Tyagi AK (2017) Reactive oxygen species (ROS) and cancer: Role of antioxidative nutraceuticals. Cancer Lett 387:95–105
Rasoulpour RJ, Terry C, LeBaron MJ, Stebbins K, Ellis-Hutchings RG, Billington R (2014) Mode-of-action and human relevance framework analysis for rat Leydig cell tumors associated with sulfoxaflor. Crit Rev Toxicol 2:25–44
Redza-Dutordoir M, Averill-Bates DA (2016) Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta 12:2977–2992
Ryter SW, Kim HP, Hoetzel A, Park JW, Nakahira K, Wang X, Choi AM (2007) Mechanisms of cell death in oxidative stress. Antioxid Redox Signal 9(1):49–89
Saleh AM, Vijayasarathy C, Masoud L (2003) Paraoxon apoptosis in eL4 cells via activation of mitochondrial pathways. Toxicol Appl Pharmacol 90:47
Sanjeewa KA, Kang N, Ahn G, Jee Y, Kim YT, Jeon YJ (2018) Bioactive potentials of sulfated polysaccharides isolated from brown seaweed Sargassum spp in related to human health applications: A review. Food Hydrocoll 81:200–208
Senthilkumar K, Manivasagan P, Venkatesan J, Kim SK (2013) Brown seaweed fucoidan: biological activity and apoptosis, growth signaling mechanism in cancer. Int J Biol Macromol 60:366–374
Senthilkumar K, Ramajayam G, Venkatesan J, Kim S, Ahn B-C (2017) Biomedical applications of fucoidan, seaweed polysaccharides. In: Sukumaran A, Kim S-K (eds) Venkatesan J. Seaweed polysaccharides Elsevier Inc., USA, pp 269–281
Sezer AD, Hatipoğlu F, Cevher E, Oğurtan Z, Baş AL, Akbuğa J (2007) Chitosan film containing fucoidan as a wound dressing for dermal burn healing: preparation and in vitro/in vivo evaluation. AAPS Pharm Sci Tech 8(2):39
Shi Y (2002) Mechanisms of caspase inhibition and activation during apoptosis. Mol Cell 9:459–470
Shi Y (2004) Caspase activation, inhibition, and reactivation: a mechanistic view. Protein Sci 13(8):1979–1987
Simon-Delso N, Amaral-Rogers V, Belzunces LP, Bonmatin JM, Chagnon M, Downs C, Furlan L, Gibbons DW, Giorio C, Girolami V, Goulson D, Kreutzweiser DP, Krupke CH, Liess M, Long E, McField M, Mineau P, Mitchell EA, Morrissey CA, Noome DA, Pisa L, Settele J, Stark JD, Tapparo A, Van Dyck H, Van Praagh J, Van der Sluijs JP, Whitehorn PR, Wiemers M (2015) Systemic insecticides (neonicotinoids and fipronil): trends, uses, mode of action and metabolites. Environ Sci Pollut Res Int 22(1):5–34
Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV (2019) Blood-brain barrier: from physiology to disease and back. Physiol Rev 99(1):21–78
Terayama H, Endo H, Tsukamoto H, Matsumoto K, Umezu M, Kanazawa T, Ito M, Sato T, Naito M, Kawakami S, Fujino Y, Tatemichi M, Sakabe K (2016) Acetamiprid accumulates in different amounts in murine brain regions. Int J Environ Res Public Health 13(10):937
Thomes P, Rajendran M, Pasanban B, Rengasamy R (2010) Cardioprotective activity of Cladosiphon okamuranus fucoidan against isoproterenol induced myocardial infarction in rats. Phytomedicine 18(1):52–57
Vishchuk OS, Ermakova SP, Zvyagintseva TN (2011) Sulfated polysaccharides from brown seaweeds Saccharina japonica and Undaria pinnatifida: isolation, structural characteristics, and antitumor activity. Carbohyd Res 346(17):2769–2776
Vousden KH, Lu X (2002) Live or let die: the cells response to p53. Nat Rev Cancer 2(8):594–604
Wang CY, Wu TC, Hsieh S L, Tsai YH, Yeh C W, Huang CY (2015) Antioxidant activity and growth inhibition of human colon cancer cells by crude and purified fucoidan preparations extracted from Sargassum cristaefolium. J Food Drug Anal 23(4):766–777
Wang J, Hu S, Nie S, Yu Q, Xie M (2016) Reviews on mechanisms of in vitro antioxidant activity of polysaccharides. Oxid Med Cell Longev 2016:5692852
Wang J, Zhang Q, Jin W, Niu X, Zhang H (2011) Effects and mechanism of low molecular weight fucoidan in mitigating the peroxidative and renal damage induced by adenine. Carbohydr Polym 84:417–423
Wang T, Ma M, Chen C, Yang X, Qian Y (2021) Three widely used pesticides and their mixtures induced cytotoxicity and apoptosis through the ROS-related caspase pathway in HepG2 cells. Food Chem Toxicol 152:112162
Wang X (2001) The expanding role of mitochondria in apoptosis. Genes Dev 15(22):2922–2933
Wang X, Anadón A, Wu Q, Qiao F, Ares I, Martínez-Larrañaga MR, Yuan Z, Martínez MA (2018) Mechanism of neonicotinoid toxicity: impact on oxidative stress and metabolism. Annu Rev Pharmacol Toxicol 58:471–507
Wang X, Martínez MA, Dai M, Chen D, Ares I, Romero A, Castellano V, Martínez M, Rodríguez JL, Martínez-Larrañaga MR, Anadón A, Yuan Z (2016) Permethrin-induced oxidative stress and toxicity and metabolism A review. Environ Res 149:86–104
Wang Y, Xing M, Cao Q, Ji A, Liang H, Song S (2019) Biological activities of fucoidan and the factors mediating ıts therapeutic effects: a review of recent studies. Mar Drugs 17(3):183
Wills E (1966) Mechanisms of lipid peroxide formation in animal tissues. Biochem J 99(3):667
Wood TJ, Goulson D (2017) The environmental risks of neonicotinoid pesticides: a review of the evidence post 2013. Environ Sci Pollut Res Int 24(21): 17285–17325
Xue CH, Fang Y, Lin H, Chen L, Li ZJ, Deng D, Lu CX (2001) Chemical characters and antioxidative properties of sulfated polysaccharides from Laminaria japonica. J Appl Phycol 13(1):67–70
Yamasaki-Miyamoto Y, Yamasaki M, Tachibana H, Yamada K (2009) Fucoidan induces apoptosis through activation of caspase-8 on human breast cancer MCF-7 cells. J Agric Food Chem 57(18):8677–8682
Young A, Kang JH, Kang DJ, Venkatesan J, Chang HK, Bhatnagar I, Chang KY, Hwang JH, Salameh Z, Kim SK, Kim HT, Kim DG (2016) Interaction of stem cells with nano hydroxyapatite-fucoidan bionanocomposites for bone tissue regeneration. Int J Biol Macromol 93(Pt B):1488–1491
Zhang HG, Wang J, Yang X, Hsu HC, Mountz JD (2004) Regulation of apoptosis proteins in cancer cells by ubiquitin. Oncogene 23(11):2009–2015
Zhang JH (2011) Oxidative stress: role in acetamiprid induced impairment of the male mice reproductive system. Agric Sci China 10:786–796
Zhu Y, Loso MR, Watson GB, Sparks TC, Rogers RB, Huang JX, Gerwick BC, Babcock JM, Kelley D, Hegde VB, Nugent BM, Renga JM, Denholm I, Gorman K, DeBoer GJ, Hasler J, Meade T, Thomas JD (2011) Discovery and characterization of sulfoxaflor, a novel insecticide targeting sap-feeding pests. J Agric Food Chem 59(7):2950–2957
Acknowledgements
The authors would like to thank Prof.Dr Mehmet Bertan Yılmaz from the Department of Medical Biology, Faculty of Medicine, Cukurova University, for his valuable contribution to molecular analyzes. The authors also would like to thank from the Enzymology Laboratory and Biotechnology Laboratory, Department of Food Engineering, and Biotechnology Research and Application Center, Cukurova University.
Funding
The financial support for this project (FYL-2019–12176) from Cukurova University Scientific Research Commission is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
PPB Conceptualization. PPB Funding acquisition. PPB and MK, CC Investigation. PPB and MK Project administration. PPB Supervision, PPB Writing — original draft. PPB, MK, CC Writing — review and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All the experiments were conducted in accordance with the protocols approved by Ethics Committee of the Cukurova University Faculty of Medicine Experimental Medicine Research and Application Center (Approval Code: 4, Approval Date: 08.07.2019).
Consent to Participate
Not applicable.
Consent for Publication
All authors consented to the publication of this research article.
Conflict of Interest
The authors declare no competing interests
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Piner Benli, P., Kaya, M. & Coskun, C. Fucoidan Modulated Oxidative Stress and Caspase-3 mRNA Expression Induced by Sulfoxaflor in the Brain of Mice. Neurotox Res 39, 1908–1919 (2021). https://doi.org/10.1007/s12640-021-00415-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-021-00415-0