Abstract
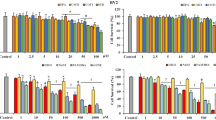
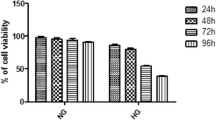
Cellular redox dysregulation produced by aldose reductase (AR) in the presence of high blood sugar is a mechanism involved in neurodegeneration commonly observed in diabetes mellitus (DM) and Parkinson’s disease (PD); therefore, AR is a key target for treatment of both diseases. The substituted triazinoindole derivatives 2-(3-thioxo-2H-[1,2,4]triazino[5,6-b]indol-5(3H)-yl) acetic acid (cemtirestat or CMTI) and 2-(3-oxo-2H-[1,2,4]triazino[5,6-b]indol-5(3H)-yl) acetic acid (COTI) are well-known AR inhibitors (ARIs). The neuroprotective properties of CMTI, COTI, the clinically used epalrestat (EPA), and the pyridoindole antioxidants stobadine and SMe1EC2 were all tested in the neurotoxic models produced by hyperglycemic glucotoxicity (HG, 75 mM d-glucose, 72 h), 6-hydroxydopamine (6-OHDA), and HG+6-OHDA models in PC12 cells. Cell viability decreased in all toxic models, increased by 1–5 μM EPA, and decreased by COTI at ≥ 2.5 μM. In the HG model alone, where compounds were present in the medium for 24 h after a continuous 24-h exposure to HG, cell viability was improved by 100 nM–5 μM EPA, 1–10 μM ARIs, and the antioxidants studied, but decreased by EPA at ≥ 10 μM. In the 6-OHDA model alone, where cells were treated with compounds for 24 h and further exposed to 100 μM 6-OHDA (8 h), only the antioxidants protected cell viability. In the HG+6-OHDA model, where cells were treated with all compounds (1 nM to 50 μM) for 48 h and exposed to 75 mM glucose for 24 h followed by incubation with 6-OHDA for 8 h, cell viability was protected by 100 nM–10 μM ARIs and 100–500 nM EPA, but not by antioxidants. All ARIs inhibited the HG+6-OHDA-induced increase in iNOS, IL-1β, TNF-α, 3-NT, and total oxidant status at 1–50 μM, while increased SOD, CAT, GPx, and total antioxidant status at 1–10 μM. EPA and CMTI also reduced the HG+6-OHDA-induced increase in the cellular levels of nuclear factor kB (NF-KB). The neuroprotective potential of the novel ARIs and the pyridoindole antioxidants studied constitutes a promising tool for the development of therapeutic strategies against DM-induced and PD-related neurodegeneration.







Similar content being viewed by others
References
Amiri E, Ghasemi R, Moosavi M (2016) Agmatine protects against 6-OHDA-induced apoptosis, and ERK and Akt/GSK disruption in SH-SY5Y cells. Cell Mol Neurobiol 36:829–838
Ashraghi MR, Pagano G, Polychronis S, Niccolini F, Politis M (2016) Parkinson';s disease, diabetes and cognitive impairment. Recent Pat Endocr Metab Immune Drug Discov 10(1):11–21
Baba M, Kimura K, Suda T, Yagihashi S, Aomori Diabetic Study (ADNS) Group (2006) Three-year inhibition of aldose reductase on development of symptomatic neuropathy in diabetic patients. J Peripher Nerv Syst 11:176–178
Balcerczyk A, Bartosz G, Drzewinska J, Piotrowski Ł, Pulaski Ł, Stefek M (2014) Antioxidant action of SMe1EC2, the low-basicity derivative of the pyridoindole stobadine, in cell free chemical models and at cellular level. Interdiscip Toxicol 7:27–32
Bali EB, Ergin V, Rackova L, Bayraktar O, Küçükboyaci N, Karasu C (2014) Olive leaf extracts protect cardiomyocytes against 4-hydroxynonenal-induced toxicity in vitro: comparison with oleuropein, hydroxytyrosol, and quercetin. Planta Med 80:984–992
Bergantin LB (2019) Diabetes and Parkinson’s disease: debating the link through Ca2+/cAMP signalling. Curr Diabetes Rev 16:238–241. https://doi.org/10.2174/1573399815666190711113644
Biosa A, Outeiro TF, Bubacco L, Bisaglia M (2018) Diabetes mellitus as a risk factor for Parkinson’s disease: a molecular point of view. Mol Neurobiol 55:8754–8763
Cherbuin N, Walsh EI (2019) Sugar in mind: untangling a sweet and sour relationship beyond type 2 diabetes. Front Neuroendocrinol 54:100769
Chung SS, Ho EC, Lam KS, Chung SK (2003) Contribution of polyol pathway to diabetes-induced oxidative stress. J Am Soc Nephrol 14:S233–S236
Cumaoglu A, Cevik C, Rackova L, Ari N, Karasu C (2007) Effects of antioxidant stobadine on protein carbonylation, advanced oxidation protein products and reductive capacity of liver in streptozotocin-diabetic rats: role of oxidative/nitrosative stress. Biofactors 30:171–178
Cumaoglu A, Stefek M, Bauer V, Ari N, Aricioglu A, Karasu C (2010) Glycoxidative and nitrosative stress in kidney of experimental diabetic rats: effects of the prydoindole antioxidant stobadine. Neuro Endocrinol Lett 31:313–318
Di Domenico F, Tramutola A, Butterfield DA (2017) Role of 4-hydroxy-2-nonenal (HNE) in the pathogenesis of alzheimer disease and other selected age-related neurodegenerative disorders. Free Radic Biol Med 111:253–261
Elmazoglu Z, Ergin V, Sahin E, Kayhan H, Karasu C (2017) Oleuropein and rutin protect against 6-OHDA-induced neurotoxicity in PC12 cells through modulation of mitochondrial function and unfolded protein response. Interdiscip Toxicol 10:129–141
Elmazoglu Z, Yar Saglam AS, Sonmez C, Karasu C (2018) Luteolin protects microglia against rotenone-induced toxicity in a hormetic manner through targeting oxidative stress response, genes associated with Parkinson’s disease and inflammatory pathways. Drug Chem Toxicol 12:1–8
Emekli-Alturfan E (2009) A novel automated method to measure total antioxidant response against potent free radical reactions. J Clin Lab Anal 23:93–98
Erel O (2004) A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem 37:112–119
Erel O (2005) A new automated colorimetric method for measuring total oxidant status. Clin Biochem 38:1103–1111
Farzam A, Chohan K, Strmiskova M, Hewitt SJ, Park DS, Pezacki JP, Özcelik D (2019) A functionalized hydroxydopamine quinone links thiol modification to neuronal cell death. Redox Biol 28:101377
Gao K, Liu M, Cao J, Yao M, Lu Y, Li J, Zhu X, Yang Z, Wen A (2014) Protective effects of Lycium barbarum polysaccharide on 6-OHDA-induced apoptosis in PC12 cells through the ROS-NO pathway. Molecules 20:293–308
Gugliucci A (2017) Formation of fructose-mediated advanced glycation end products and their roles in metabolic and inflammatory diseases. Adv Nutr 8:54–62
Güneş A, Ceylan A, Sarioglu Y, Stefek M, Bauer V, Karasu C, Antioxidants in Diabetes-Induced Complications (ADIC) Study Group (2005) Reactive oxygen species mediate abnormal contractile response to sympathetic nerve stimulation and noradrenaline in the vas deferens of chronically diabetic rats: effects of in vivo treatment with antioxidants. Fundam Clin Pharmacol 19:73–79
Hlavac M, Kovacikova L, Soltesova Prnova M, Šramel P, Addova G, Majekova M, Hanquet G, Bohac A, Stefek M (2019) Development of novel oxotriazinoindole inhibitors of aldose reductase: isosteric sulfur/oxygen replacement in the thioxotriazinoindole cemtirestat markedly improved inhibition selectivity. J Med Chem 63:369–381. https://doi.org/10.1021/acs.jmedchem.9b01747
Hu LW, Yen JH, Shen YT, Wu KY, Wu MJ (2014) Luteolin modulates 6-hydroxydopamine-induced transcriptional changes of stress response pathways in PC12 cells. PLoS One 9:e97880
Hwang SH, Kim H-Y, Guillen Quispe YN, Wang Z, Zuo G, Lim SS (2019) Aldose reductase, protein glycation inhibitory and antioxidant of Peruvian Medicinal Plants: the case of Tanacetum parthenium L. and its constituents. Molecules 24(10):2010
Iyer S, Sam FS, DiPrimio N, Preston G, Verheijen J, Murthy K, Parton Z, Tsang H, Lao J, Morava E, Perlstein EO (2019) Repurposing the aldose reductase inhibitor and diabetic neuropathy drug epalrestat for the congenital disorder of glycosylation PMM2-CDG. Dis Model Mech 12:dmm040584. https://doi.org/10.1242/dmm.040584
Jellinger K, Linert L, Kienzl E, Herlinger E, Youdim MB (1995) Chemical evidence for 6-hydroxydopamine to be an endogenous toxic factor in the pathogenesis of Parkinson’s disease. J Neural Transm Suppl 46:297–314
Jiang X, Wang X, Tuo M, Ma J, Xie A (2018) RAGE and its emerging role in the pathogenesis of Parkinson’s disease. Neurosci Lett 672:65–69
Juranek I, Horakova L, Rackova L, Stefek M (2010) Antioxidants in treating pathologies involving oxidative damage: an update on medicinal chemistry and biological activity of stobadine and related pyridoindoles. Curr Med Chem 17:552–570
Kalvala A, Yerra VG, Kumar A (2019) LONP1 induction by SRT1720 attenuates mitochondrial dysfunction against high glucose induced neurotoxicity in PC12 cells. Toxicol in Vitro 19:104695
Kang D, Hamasaki N (2005) Alterations of mitochondrial DNA in common diseases and disease states: aging, neurodegeneration, heart failure, diabetes, and cancer. Curr Med Chem 12:429–441
Karasu C (2010) Glycoxidative stress and cardiovascular complications in experimentally-induced diabetes: effects of antioxidant treatment. Open Cardiovasc Med J 4:240–256
Karasu C, Cumaoğlu A, Gürpinar AR, Kartal M, Kovacikova L, Milackova I, Stefek M (2012) Aldose reductase inhibitory activity and antioxidant capacity of pomegranate extracts. Interdiscip Toxicol 5:15–20
Koutsopoulos K, Lavrentaki V, Antoniou I, Kousaxidis A, Lefkopoulou M, Tsantili-Kakoulidou A, Kovacikova L, Stefek M, Nicolaou I (2020) Design synthesis and evaluation of novel aldose reductase inhibitors: the case of indolyl-sulfonyl-phenols. Bioorg Med Chem 28:115575
Li QR, Wang Z, Zhou W, Fan SR, Ma R, Xue L, Yang L, Li YS, Tan HL, Shao QH, Yang HY (2016) Epalrestat protects against diabetic peripheral neuropathy by alleviating oxidative stress and inhibiting polyol pathway. Neural Regen Res 11:345–351
Mousavi SH, Tayarani NZ, Parsaee H (2010) Protective effect of saffron extract and crocin on reactive oxygen species-mediated high glucose-induced toxicity in PC12 cells. Cell Mol Neurobiol 30:185–191
Msibi ZNP, Mabandla MV (2019) Oleanolic acid mitigates 6-hydroxydopamine neurotoxicity by attenuating intracellular ROS in PC12 cells and striatal microglial activation in rat brains. Front Physiol 10:1059
Mule NK, Singh JN (2018) Diabetes mellitus to teurodegenerative disorders: is oxidative stress fueling the flame? CNS Neurol Disord Drug Targets 17:644–653
Nakayama M, Nakamura J, Hamada Y, Chaya S, Mizubayashi R, Yasuda Y, Kamiya H, Koh N, Hotta N (2001) Aldose reductase inhibition ameliorates pupillary light reflex and F-wave latency in patients with mild diabetic neuropathy. Diabetes Care 24:1093–1098
Pagano G, Polychronis S, Wilson H, Giordano B, Ferrara N, Niccolini F, Politis M (2018) Diabetes mellitus and Parkinson disease. Neurology 90:e1654–e1662
Pekiner B, Ulusu NN, Das-Evcimen N, Sahilli M, Aktan F, Stefek M, Stolc S, Karasu C (2002) Antioxidants in diabetes-induced complications study group. In vivo treatment with stobadine prevents lipid peroxidation, protein glycation and calcium overload but does not ameliorate Ca2+-ATPase activity in heart and liver of streptozotocin-diabetic rats: comparison with vitamin E. Biochim Biophys Acta 1588:71–78
Prnova MS, Kovacikova L, Svik K, Bezek S, Elmazoğlu Z, Karasu C, Stefek M (2020) Triglyceride-lowering effect of the aldose reductase inhibitor cemtirestat—another factor that may contribute to attenuation of symptoms of peripheral neuropathy in STZ-diabetic rats. Naunyn Schmiedeberg’s Arch Pharmacol 393:651–661
Račková L, Stefek M, Majekova M (2002) Structural aspects of antioxidant activity of substituted pyridoindoles. Redox Rep 7:207–214
Račková L, Cumaoğlu A, Bağrıacık EU, Štefek M, Maechler P, Karasu C (2011) Novel hexahydropyridoindole derivative as prospective agent against oxidative damage in pancreatic β cells. Med Chem 7:711–717
Ramazani E, Tayarani-Najaran Z, Fereidoni M (2019) Celecoxib, indomethacin, and ibuprofen prevent 6-hydroxydopamine-induced PC12 cell death through the inhibition of NFκB and SAPK/JNK pathways. Iran J Basic Med Sci 22:477–484
Rayegan S, Dehpour AR, Sharifi AM (2017) Studying neuroprotective effect of Atorvastatin as a small molecule drug on high glucose-induced neurotoxicity in undifferentiated PC12 cells: role of NADPH oxidase. Metab Brain Dis 32:41–49
Saberi Firouzi S, Namazi Sarvestani N, Bakhtiarian A, Ghazi Khansari M, Karimi MY, Ranjbar A, Safa M, Hosseini A (2018) Sildenafil protective effects on high glucose-induced neurotoxicity in PC12 cells: the role of oxidative stress, apoptosis, and inflammation pathways in an in vitro cellular model for diabetic neuropathy. Neurol Res 40:624–636
Sakul A, Cumaoğlu A, Aydin E, Ari N, Dilsiz N, Karasu C (2013) Age- and diabetes-induced regulation of oxidative protein modification in rat brain and peripheral tissues: consequences of treatment with antioxidant pyridoindole. Exp Gerontol 48:476–484
Şakul A, Arı N, Sotnikova R, Ozansoy G, Karasu C (2018) A pyridoindole antioxidant SMe1EC2 regulates contractility, relaxation ability, cation channel activity, and protein-carbonyl modifications in the aorta of young and old rats with or without diabetes mellitus. Geroscience 40:377–392
Santiago JA, Potashkin JA (2013) Shared dysregulated pathways lead to Parkinson’s disease and diabetes. Trends Mol Med 19:176–186
Schober A (2004) Classic toxin-induced animal models of Parkinson’s disease: 6-OHDA and MPTP. Cell Tissue Res 318:215–224
Shukla K, Pal PB, Sonowal H, Srivastava SK, Ramana KV (2017) Aldose reductase inhibitor protects against hyperglycemic stress by activating Nrf2-dependent antioxidant proteins. J Diabetes Res 2017:6785852
Skalska S, Kyselova Z, Gajdosikova A, Karasu C, Stefek M, Stolc S (2008) Protective effect of stobadine on NCV in streptozotocin-diabetic rats: augmentation by vitamin E. Gen Physiol Biophys 27:106–114
Soltesova Prnova M, Ballekova J, Gajdosikova A, Gajdosik A, Stefek M (2015) A novel carboxymethylated mercaptotriazinoindole inhibitor of aldose reductase interferes with the polyol pathway in streptozotocin-induced diabetic rats. Physiol Res 64:587–591
Soltesova Prnova M, Svik K, Bezek S, Kovacikova L, Karasu C, Stefek M (2019) 3-Mercapto-5H-1,2,4-triazino[5,6-b]Indole-5-acetic acid (cemtirestat) alleviates symptoms of peripheral diabetic neuropathy in Zucker diabetic fatty (ZDF) rats: a role of aldose reductase. Neurochem Res 44:1056–1064
Stefek M, Milackova I, Juskova-Karasova M, Snirc V (2013) Antioxidant action of the hexahydropyridoindole SMe1EC2 in the cellular system of isolated red blood cells in vitro. Redox Rep 18:71–75
Stefek M, Soltesova Prnova M, Majekova M, Rechlin C, Heine A, Klebe G (2015) Identification of novel aldose reductase inhibitors based on carboxymethylated mercaptotriazinoindole scaffold. J Med Chem 58:2649–2657
Stefek M, Soltesova Prnova M, Ballekova J, Majekova M (2016) Cemtirestat, a novel aldose reductase inhibitor and antioxidant, in multitarget pharmacology of diabetic complications. Int J Adv Sci Eng Technol 4:41–44
Sultan CS, Saackel A, Stank A, Fleming T, Fedorova M, Hoffmann R, Wade RC, Hecker M, Wagner AH (2018) Impact of carbonylation on glutathione peroxidase-1 activity in human hyperglycemic endothelial cells. Redox Biol 16:113–122
Sun Y, Chang YH, Chen HF, Su YH, Su HF, Li CY (2012) Risk of Parkinson disease onset in patients with diabetes: a 9-year population-based cohort study with age and sex stratifications. Diabetes Care 35:1047–1049
Ulusu NN, Sahilli M, Avci A, Canbolat O, Ozansoy G, Ari N, Bali M, Stefek M, Stolc S, Gajdosik A, Karasu C (2003) Pentose phosphate pathway, glutathione-dependent enzymes and antioxidant defense during oxidative stress in diabetic rodent brain and peripheral organs: effects of stobadine and vitamin E. Neurochem Res 28:815–823
Ulusu NN, Gök M, Sayin Şakul AA, Ari N, Stefek M, Karasu C, The ADIC (Antioxidants in Diabetes-Induced Complications) Study Group (2017) Antioxidant SMe1EC2 modulates pentose phosphate pathway and glutathione-dependent enzyme activities in tissues of aged diabetic rats. Interdiscip Toxicol 10:148–154
Umeno A, Biju V, Yoshida Y (2017) In vivo ROS production and use of oxidative stress-derived biomarkers to detect the onset of diseases such as Alzheimer’s disease, Parkinson’s disease, and diabetes. Free Radic Res 51:413–427
Vicente Miranda H, El-Agnaf OM, Outeiro TF (2016) Glycation in Parkinson’s disease and Alzheimer’s disease. Mov Disord 31:782–790
Woltjer RL, Maezawa I, Ou JJ, Montine KS, Montine TJ (2003) Advanced glycation endproduct precursor alters intracellular amyloid-beta/A beta PP carboxy-terminal fragment aggregation and cytotoxicity. J Alzheimers Dis 5:467–476
Yagihashi S (2016) Glucotoxic mechanisms and related therapeutic approaches. Int Rev Neurobiol 127:121–149
Yang YW, Hsieh TF, Li CI, Liu CS, Lin WY, Chiang JH, Li TC, Lin CC (2017) Increased risk of Parkinson disease with diabetes mellitus in a population-based study. Medicine (Baltimore) 96:e5921
Yeung PKK, Lai AKW, Son HJ, Zhang X, Hwang O, Chung SSM, Chung SK (2017) Aldose reductase deficiency leads to oxidative stress-induced dopaminergic neuronal loss and autophagic abnormality in an animal model of Parkinson’s disease. Neurobiol Aging 50:119–133
Yülek F, Or M, Ozoğul C, Isik AC, Ari N, Stefek M, Bauer V, Karasu C (2007) Effects of stobadine and vitamin E in diabetes-induced retinal abnormalities: involvement of oxidative stress. Arch Med Res 38:503–511
Zhou L, Cheng Y (2019) Alpha-lipoic acid alleviated 6-OHDA-induced cell damage by inhibiting AMPK/mTOR mediated autophagy. Neuropharmacology 155:98–103
Funding
This work was supported by The Scientific and Technological Research Council of Turkey (TUBITAK Project No: 215S197; SAS-TUBITAK JRP 2015/7) and the Slovak Research and Development Agency (Contract No. APVV-15-0455, VEGA 2/0005/2018).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights Informed Consent
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Elmazoglu, Z., Prnova, M.S., Santamaria, A. et al. Combatting Nitrosative Stress and Inflammation with Novel Substituted Triazinoindole Inhibitors of Aldose Reductase in PC12 Cells Exposed to 6-Hydroxydopamine Plus High Glucose. Neurotox Res 39, 210–226 (2021). https://doi.org/10.1007/s12640-020-00305-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-020-00305-x