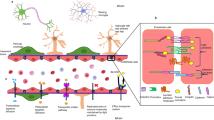
Abstract
Major depressive disorders (MDD) are often comorbid with the gastrointestinal (GI) disorders. Brain-derived neurotrophic factor precursor (proBDNF) has been reported to contribute to the development of depression in mouse models. However, the role of proBDNF in depression-associated GI disorders is still unrevealed. Mice experienced unpredictable chronic mild stress (UCMS) procedure and were then intraperitoneally injected with fluoxetine (20 mg/kg). Open field test (OFT), forced swimming test (FST), and sucrose preference test (SPT) were performed to evaluate the severity of depression. Oral administration of food dye gel and histological staining were performed to assess GI transit and morphological alterations. QPCR was performed to assess the mRNA levels of inflammatory cytokines. Additionally, flow cytometry, immunohistochemistry, and immunofluorescence were performed to examine the expression and cellular localization of proBDNF. It was found that (a) in the peripheral blood, the expression of proBDNF and its receptor pan neurotrophin receptor 75 (p75NTR) in CD11b+ cells in depressive mice was higher than in controls; (b) the GI motility was decreased after the UCMS procedure and partly reversed by fluoxetine treatment; (c) proBDNF/p75NTR was highly expressed in macrophages in the intestinal lamina propria; (d) the upregulated proBDNF/p75NTR and the activated cytokines, including IL (interleukin)-1β, IL-6, IL-10, and IFN (interferon)-γ, were positively correlated with the depression and GI disorders, and were inhibited by fluoxetine treatment. UCMS procedure upregulated the expression of proBDNF and p75NTR in monocytes/macrophages of peripheral blood and intestinal lamina propria, which may be involved in the pathogenesis of depression-associated GI disorders. Fluoxetine reversed the GI dysfunction, infiltration of macrophages, and upregulation of proBDNF signaling in the depressive mice.






Similar content being viewed by others
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Change history
19 September 2020
Dr. Chang-Qi Li should be added as co-author because Fig. 1 originated from him.
References
Bai YY, Ruan CS, Yang CR, Li JY, Kang ZL, Zhou L, Liu D, Zeng YQ, Wang TH, Tian CF, Liao H, Bobrovskaya L, Zhou XF (2016) ProBDNF signaling regulates depression-like behaviors in rodents under chronic stress. Neuropsychopharmacology 41:2882–2892
Ballou S, Katon J, Singh P, Rangan V, Lee HN, McMahon C, Iturrino J, Lembo A, Nee J (2019) Chronic diarrhea and constipation are more common in depressed individuals. Clin Gastroenterol Hepatol 17:2696–2703
Bassotti G (2000) Motions and emotions: the treatment of depression causes constipation. Neurogastroenterol Motil 12:113–115
Blochl A, Blochl R (2007) A cell-biological model of p75NTR signaling. J Neurochem 102:289–305
Chen JX, Pan H, Rothman TP, Wade PR, Gershon MD (1998) Guinea pig 5-HT transporter: cloning, expression, distribution, and function in intestinal sensory reception. Am J Phys 275:G433–G448
Chen F, Yu Y, Wang P, Dong Y, Wang T, Zuo X, Li Y (2014a) Brain-derived neurotrophic factor accelerates gut motility in slow-transit constipation. Acta Physiol (Oxford) 212:226–238
Chen F, Yu Y, Wang P, Dong Y, Wang T, Zuo X, Li Y (2014b) Brain-derived neurotrophic factor accelerates gut motility in slow-transit constipation. Acta Physiol (Oxford) 212:226–238
Cole SW, Conti G, Arevalo JM, Ruggiero AM, Heckman JJ, Suomi SJ (2012) Transcriptional modulation of the developing immune system by early life social adversity. Proc Natl Acad Sci U S A 109:20578–20583
Collins SM, Surette M, Bercik P (2012) The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol 10:735–742
Cryan JF, Dinan TG (2012) Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 13:701–712
De Palma G, Collins SM, Bercik P, Verdu EF (2014) The microbiota-gut-brain axis in gastrointestinal disorders: stressed bugs, stressed brain or both? J Physiol London 592:2989–2997
De Schepper S et al (2019) Self-maintaining gut macrophages are essential for intestinal homeostasis. Cell 176:676
Dickson EJ, Heredia DJ, Smith TK (2010) Critical role of 5-HT1A, 5-HT3, and 5-HT7 receptor subtypes in the initiation, generation, and propagation of the murine colonic migrating motor complex. Am J Physiol Gastrointest Liver Physiol 299:G144–G157
Diniz C, Casarotto PC, Resstel L, Joca SRL (2018) Beyond good and evil: a putative continuum-sorting hypothesis for the functional role of proBDNF/BDNF-propeptide/mBDNF in antidepressant treatment. Neurosci Biobehav Rev 90:70–83
Dipnall JF, Pasco JA, Berk M, Williams LJ, Dodd S, Jacka FN, Meyer D (2016) Into the bowels of depression: unravelling medical symptoms associated with depression by applying machine-learning techniques to a community based population sample. PLoS One 11:e0167055
Dusedau HP et al (2019) p75(NTR) regulates brain mononuclear cell function and neuronal structure in Toxoplasma infection-induced neuroinflammation. Glia 67:193–211
Fan YJ, Wu LL, Li HY, Wang YJ, Zhou XF (2008) Differential effects of pro-BDNF on sensory neurons after sciatic nerve transection in neonatal rats. Eur J Neurosci 27:2380–2390
Foster J, Neufeld KA (2014) Gut-brain axis: how the microbiome influences anxiety and depression. Int J Neuropsychopharmacol 17:27–27
Gibon J, Buckley SM, Unsain N, Kaartinen V, Seguela P, Barker PA (2015) proBDNF and p75NTR control excitability and persistent firing of cortical pyramidal neurons. J Neurosci 35:9741–9753
Guignabert C, Raffestin B, Benferhat R, Raoul W, Zadigue P, Rideau D, Hamon M, Adnot S, Eddahibi S (2005) Serotonin transporter inhibition prevents and reverses monocrotaline-induced pulmonary hypertension in rats. Circulation 111:2812–2819
Hosseinzadeh ST, Poorsaadati S, Radkani B, Forootan M (2011) Psychological disorders in patients with chronic constipation. Gastroenterol Hepatol Bed Bench 4:159–163
Hu Z, Cui Y, Qiao X, He X, Li F, Luo C, Wang S, Li C, Dai R (2018) Silencing miR-150 ameliorates experimental autoimmune encephalomyelitis. Front Neurosci 12:465
Israelyan N et al (2019) Effects of serotonin and slow-release 5-hydroxytryptophan on gastrointestinal motility in a mouse model of depression. Gastroenterology 157:507–521 e504
Jacobsen JPR, Krystal AD, Krishnan KRR, Caron MG (2016) Adjunctive 5-hydroxytryptophan slow-release for treatment-resistant depression: clinical and preclinical rationale. Trends Pharmacol Sci 37:933–944
Kalkman HO, Feuerbach D (2016) Antidepressant therapies inhibit inflammation and microglial M1-polarization. Pharmacol Ther 163:82–93
Kappelmann N, Lewis G, Dantzer R, Jones PB, Khandaker GM (2018) Antidepressant activity of anti-cytokine treatment: a systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol Psychiatry 23:335–343
Kelly JR, Borre Y, O' Brien C, Patterson E, el Aidy S, Deane J, Kennedy PJ, Beers S, Scott K, Moloney G, Hoban AE, Scott L, Fitzgerald P, Ross P, Stanton C, Clarke G, Cryan JF, Dinan TG (2016) Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res 82:109–118
Kohler CA et al (2017) Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand 135:373–387
Koloski NA, Jones M, Wai R, Gill RS, Byles J, Talley NJ (2013) Impact of persistent constipation on health-related quality of life and mortality in older community-dwelling women. Am J Gastroenterol 108:1152–1158
Kon R, Ikarashi N, Hayakawa A, Haga Y, Fueki A, Kusunoki Y, Tajima M, Ochiai W, Machida Y, Sugiyama K (2015) Morphine-induced constipation develops with increased aquaporin-3 expression in the colon via increased serotonin secretion. Toxicol Sci 145:337–347
Kopschina Feltes P, Doorduin J, Klein HC, Juarez-Orozco LE, Dierckx RA, Moriguchi-Jeckel CM, de Vries EF (2017) Anti-inflammatory treatment for major depressive disorder: implications for patients with an elevated immune profile and non-responders to standard antidepressant therapy. J Psychopharmacol 31:1149–1165
Li XQ, Wang HM, Yang CG, Zhang XH, Han DD, Wang HL (2011) Fluoxetine inhibited extracellular matrix of pulmonary artery and inflammation of lungs in monocrotaline-treated rats. Acta Pharmacol Sin 32:217–222
Li CP, Ling C, Biancani P, Behar J (2012) Effect of progesterone on colonic motility and fecal output in mice with diarrhea. Neurogastroenterol Motil 24:392–e174
Luo C, Zhong XL, Zhou FH, Li JY, Zhou P, Xu JM, Song B, Li CQ, Zhou XF, Dai RP (2016) Peripheral brain derived neurotrophic factor precursor regulates pain as an inflammatory mediator. Sci Rep 6:27171
Mardini HE, Kip KE, Wilson JW (2004) Crohn’s disease: a two-year prospective study of the association between psychological distress and disease activity. Dig Dis Sci 49:492–497
Margolis KG, Gershon MD (2016) Enteric neuronal regulation of intestinal inflammation. Trends Neurosci 39:614–624
Masand PS, Gupta S (1999) Selective serotonin-reuptake inhibitors: an update. Harv Rev Psychiatry 7:69–84
Mead E et al (2016) Drug interventions for the treatment of obesity in children and adolescents. Cochrane Database Syst Rev 11:CD012436
Meyer JH (2017) Neuroprogression and immune activation in major depressive disorder. Mod Trends Pharmacopsychiatry 31:27–36
Midenfjord I, Polster A, Sjovall H, Tornblom H, Simren M (2019) Anxiety and depression in irritable bowel syndrome: exploring the interaction with other symptoms and pathophysiology using multivariate analyses. Neurogastroenterol Motil 31:e13619
Miller AH, Raison CL (2016) The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol 16:22–34
Miller GE, Chen E, Sze J, Marin T, Arevalo JMG, Doll R, Ma R, Cole SW (2008) A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-kappaB signaling. Biol Psychiatry 64:266–272
Miller GE, Murphy MLM, Cashman R, Ma R, Ma J, Arevalo JMG, Kobor MS, Cole SW (2014) Greater inflammatory activity and blunted glucocorticoid signaling in monocytes of chronically stressed caregivers. Brain Behav Immun 41:191–199
Mowla SJ, Farhadi HF, Pareek S, Atwal JK, Morris SJ, Seidah NG, Murphy RA (2001) Biosynthesis and post-translational processing of the precursor to brain-derived neurotrophic factor. J Biol Chem 276:12660–12666
Mucida D, Esterhazy D (2018) SnapShot: gut immune niches. Cell 174(1600–1600):e1601
Muller PA, Koscsó B, Rajani GM, Stevanovic K, Berres ML, Hashimoto D, Mortha A, Leboeuf M, Li XM, Mucida D, Stanley ER, Dahan S, Margolis KG, Gershon MD, Merad M, Bogunovic M (2014) Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell 158:1210
Pang PT (2004) Cleavage of proBDNF by tPA/Plasmin is essential for long-term hippocampal plasticity. Science 306(5695):487–491
Powell ND, Sloan EK, Bailey MT, Arevalo JMG, Miller GE, Chen E, Kobor MS, Reader BF, Sheridan JF, Cole SW (2013) Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via beta-adrenergic induction of myelopoiesis. Proc Natl Acad Sci U S A 110:16574–16579
Reichardt LF (2006) Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond Ser B Biol Sci 361:1545–1564
Rief W, Nestoriuc Y, von Lilienfeld-Toal A, Dogan I, Schreiber F, Hofmann SG, Barsky AJ, Avorn J (2009) Differences in adverse effect reporting in placebo groups in SSRI and tricyclic antidepressant trials: a systematic review and meta-analysis. Drug Saf 32:1041–1056
Ruan CS, Zhou FH, He ZY, Wang SF, Yang CR, Shen YJ, Guo Y, Zhao HB, Chen L, Liu D, Liu J, Baune BT, Xiao ZC, Zhou XF (2015) Mice deficient for wild-type p53-induced phosphatase 1 display elevated anxiety- and depression-like behaviors. Neuroscience 293:12–22
Rush AJ, Fava M, Wisniewski SR, Lavori PW, Trivedi MH, Sackeim HA, Thase ME, Nierenberg AA, Quitkin FM, Kashner TM, Kupfer DJ, Rosenbaum JF, Alpert J, Stewart JW, McGrath PJ, Biggs MM, Shores-Wilson K, Lebowitz BD, Ritz L, Niederehe G, STAR*D Investigators Group (2004) Sequenced treatment alternatives to relieve depression (STAR*D): rationale and design. Control Clin Trials 25:119–142
Schechter D (1999) Estrogen, progesterone, and mood. J Gend Specif Med 2:29–36
Setiawan E, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Rajkowska G, Suridjan I, Kennedy JL, Rekkas PV, Houle S, Meyer JH (2015) Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry 72:268–275
Shen WY et al (2019) The regulatory role of ProBDNF in monocyte function: implications in Stanford type-A aortic dissection disease. FASEB J
Sherwin BB (1991) The impact of different doses of estrogen and progestin on mood and sexual behavior in postmenopausal women. J Clin Endocrinol Metab 72:336–343
Sun J, Wang F, Hu X, Yang C, Xu H, Yao Y, Liu J (2018) Clostridium butyricum attenuates chronic unpredictable mild stress-induced depressive-like behavior in mice via the gut-brain axis. J Agric Food Chem 66:8415–8421
Tan S, Wang Y, Chen K, Long Z, Zou J (2017) Ketamine alleviates depressive-like behaviors via down-regulating inflammatory cytokines induced by chronic restraint stress in mice. Biol Pharm Bull 40:1260–1267
Teng HK, Teng KK, Lee R, Wright S, Tevar S, Almeida RD, Kermani P, Torkin R, Chen ZY, Lee FS, Kraemer RT, Nykjaer A, Hempstead BL (2005) ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J Neurosci 25:5455–5463
Torres-Platas SG, Cruceanu C, Chen GG, Turecki G, Mechawar N (2014) Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav Immun 42:50–59
Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M, STAR*D Study Team (2006) Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 163:28–40
Tsai JH, Kuo CH, Yang P, Cheng KH, Wang PW, Chen CC, Hung CH (2014) Effects of antidepressants on IP-10 production in LPS-activated THP-1 human monocytes. Int J Mol Sci 15:13223–13235
Vahedi H, Merat S, Rashidioon A, Ghoddoosi A, Malekzadeh R (2005) The effect of fluoxetine in patients with pain and constipation-predominant irritable bowel syndrome: a double-blind randomized-controlled study. Aliment Pharmacol Ther 22:381–385
Wade PR, Chen J, Jaffe B, Kassem IS, Blakely RD, Gershon MD (1996) Localization and function of a 5-HT transporter in crypt epithelia of the gastrointestinal tract. J Neurosci 16:2352–2364
Waiskopf N, Ofek K, Gilboa-Geffen A, Bekenstein U, Bahat A, Bennett ER, Podoly E, Livnah O, Hartmann G, Soreq H (2014) AChE and RACK1 promote the anti-inflammatory properties of fluoxetine. J Mol Neurosci 53:306–315
Walker EA, Gelfand MD, Gelfand AN, Creed F, Katon WJ (1996) The relationship of current psychiatric disorder to functional disability and distress in patients with inflammatory bowel disease. Gen Hosp Psychiatry 18:220–229
Wang Z, Wu J-L, Zhong F, Liu Y, Yu Y-Q, Sun J-J, Wang S, Li H, Zhou X-F, Hu Z-L, Dai R-P (2019) Upregulation of proBDNF in the mesenteric lymph nodes in septic mice. Neurotox Res 36(3):540–550
Wohleb ES, McKim DB, Sheridan JF, Godbout JP (2014) Monocyte trafficking to the brain with stress and inflammation: a novel axis of immune-to-brain communication that influences mood and behavior. Front Neurosci 8:447
Wong I, Liao H, Bai X, Zaknic A, Zhong J, Guan Y, Li HY, Wang YJ, Zhou XF (2010) ProBDNF inhibits infiltration of ED1+ macrophages after spinal cord injury. Brain Behav Immun 24:585–597
Wunsch M, Jabari S, Voussen B, Enders M, Srinivasan S, Cossais F, Wedel T, Boettner M, Schwarz A, Weyer L, Göcer O, Schroeter M, Maeurer M, Woenckhaus M, Pollok K, Radbruch H, Klotz L, Scholz CJ, Nickel J, Friebe A, Addicks K, Ergün S, Lehmann PV, Kuerten S (2017) The enteric nervous system is a potential autoimmune target in multiple sclerosis. Acta Neuropathol 134:281–295
Xu Z, Shi WH, Xu LB, Shao MF, Chen ZP, Zhu GC, Hou Q (2019) Resident microglia activate before peripheral monocyte infiltration and p75NTR blockade reduces microglial activation and early brain injury after subarachnoid hemorrhage. ACS Chem Neurosci 10:412–423
Yang CR, Zhang ZG, Bai YY, Zhou HF, Zhou L, Ruan CS, Li F, Li CQ, Zheng HY, Shen LJ, Zhou XF (2014) Foraging activity is reduced in a mouse model of depression. Neurotox Res 25:235–247
Yang CR et al (2019) Antidepressant drugs correct the imbalance between proBDNF/p75NTR/sortilin and mature BDNF/TrkB in the brain of mice with chronic stress. Neurotox Res
You Z, Luo C, Zhang W, Chen Y, He J, Zhao Q, Zuo R, Wu Y (2011) Pro- and anti-inflammatory cytokines expression in rat's brain and spleen exposed to chronic mild stress: involvement in depression. Behav Brain Res 225:135–141
Zhong F et al (2019) Brain-derived neurotrophic factor precursor in the hippocampus regulates both depressive and anxiety-like behaviors in rats. Front Psychiatry:9
Zhou L, Xiong J, Lim Y, Ruan Y, Huang C, Zhu Y, Zhong JH, Xiao Z, Zhou XF (2013) Upregulation of blood proBDNF and its receptors in major depression. J Affect Disord 150:776–784
Acknowledgments
We thank Shanghai Yile Biotechnology Corp. for providing the biotin humanized monoclonal anti-proBDNF antibody.
Funding
This research was supported by National Natural Science Foundation of China (81771354 to RD, 81873770 to LH, and 81901231 to ZLH), and the Fundamental Research Funds for the Central Universities of Central South University (2019zzts812 to Yu YQ, 2019zzts1048 to Wang Z, and 506021710 to Liu Y).
Author information
Authors and Affiliations
Contributions
YY conducted the study, data collection, data analysis, and manuscript preparation; YZ conducted the study, data collection, and data analysis; ZW and YL helped do the animal-related experiments; HL helped design and analyze the data; XFZ provided reagents, interpreted data, and revised manuscripts; RD and ZH were responsible for designing and interpretation of the work, data collection, data analysis, and manuscript drafting; all authors had a final approval of the manuscript submission.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yun-qing Yu and Yan-ling Zhang are co-first authors.
Rights and permissions
About this article
Cite this article
Yu, YQ., Zhang, YL., Wang, Z. et al. Involvement of proBDNF in Monocytes/Macrophages with Gastrointestinal Disorders in Depressive Mice. Neurotox Res 38, 887–899 (2020). https://doi.org/10.1007/s12640-020-00235-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-020-00235-8