Abstract
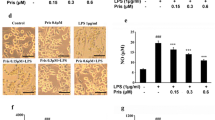
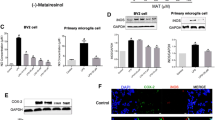
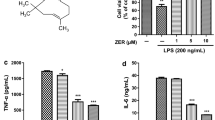
Microglia-involved neuroinflammation in the central nervous system (CNS) has been shown to aggravate brain damage and is associated with the pathogenesis of various neurodegenerative diseases. Thus, suppression of microglial activity has the potential to be a strategy for the treatment of neurodegenerative diseases. Pinitol, a methylated product of d-chiro-inositol, has been used as a treatment for blood-sugar metabolism and as an anti-tumor agent via its anti-inflammatory effects in cancer. However, whether or not pinitol can inhibit microglia-associated neuroinflammation is still unknown. This study aims to determine the effects of pinitol on inflammatory responses in BV2 microglia induced by LPS. Here, we found that the presence of pinitol ameliorates LPS-induced oxidative stress by reducing the production of ROS. Pinitol suppresses the expression and secretion of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6. Notably, pinitol prevents the production of NO and PGE2 by inhibiting the expression of iNOS and COX-2. Mechanistically, our findings demonstrate that pinitol inhibits the phosphorylation and degradation of IκBα and subsequent activation of NF-κB. Furthermore, we show that pinitol increases the expression of TREM2 in BV2 microglia, and silencing of TREM2 abolished the anti-inflammatory effects of pinitol. These findings suggest that TREM2 mediates the protective effects of pinitol against LPS in microglia. In summary, our results display that pinitol possesses a robust and beneficial effect against the LPS-induced inflammatory response in microglia.








Similar content being viewed by others
References
Aggarwal BB, Van Kuiken ME, Iyer LH, Harikumar KB, Sung B (2009) Molecular targets of nutraceuticals derived from dietary spices: potential role in suppression of inflammation and tumorigenesis. Exp Biol Med (Maywood) 234(8):825–849
Amor S, Puentes F, Baker D, van der Valk P (2010) Inflammation in neurodegenerative diseases. Immunology 129(2):154–169
Bates SH, Jones RB, Bailey CJ (2000) Insulin-like effect of pinitol. Br J Pharmacol 130(8):1944–1948
Biber K, Moller T, Boddeke E, Prinz M (2016) Central nervous system myeloid cells as drug targets: current status and translational challenges. Nat Rev Drug Discov 15(2):110–124
Block ML, Hong JS (2005a) Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol 76(2):77–98
Block ML, Hong JS (2005b) Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol 76:77–98
Brown GC (2019) The endotoxin hypothesis of neurodegeneration. J Neuroinflammation 16(1):180
Eikelenboom P, Bate C, VanGooletal WA (2002) Neuroinflammation in Alzheimer’s disease and prion disease. Glia 40(2):232–239
Erica CD, Mollie KM (2019) Cellular specificity of NF-κB function in the nervous system. Front Immunol 10:1043
Fujioka S, Niu J, Schmidt C, Sclabas GM, Peng B, Uwagawa T, Li Z, Evans DB, Abbruzzese JL, Chiao PJ (2004) NF-κB and AP-1 connection: mechanism of NF-κB-dependent regulation of AP-1 activity. Mol Cell Biol 24(17):7806–7819
Hamerman JA, Jarjoura JR, Humphrey MB, Nakamura MC, Seaman WE, Lanier LL (2006) Cutting edge: inhibition of TLR and FcR responses in macrophages by triggering receptor expressed on myeloid cells (TREM)-2 and DAP12. J Immunol 177(4):2051–2055
Joseph L (2010) D-Chiro-inositol glycans in insulin signaling and insulin resistance. Mol Med 16(11–12):543–551
Kaltschmidt B, Kaltschmidt C (2009) NF-κB in the nervous system. Cold Spring Harb Perspect Biol 1(3):a001271
Konishi H, Kiyama H (2018) Microglial TREM2/DAP12 signaling: a double-edged sword in neural diseases. Front Cell Neurosci:12–206
Kopitar JN (2015) Innate immune response in brain, NF-Kappa B signaling and cystatins. Front Mol Neurosci:8–73
Kumagai N, Chiba Y, Hosono M, Fujii M, Kawamura N, Keino H, Yoshikawa K, Ishii S, Saitoh Y, Satoh M, Shimada A, Hosokawa M (2007) Involvement of pro-inflammatory cytokines and microglia in an age-associated neurodegeneration model, the SAMP10 mouse. Brain Res 1185:75–85
Kumar A, Takada Y, Boriek AM, Aggarwal BB (2004) Nuclear factor-kB: its role in health and disease. J Mol Med 82:434–448
Lee KW, Jung SY, Choi SM, Yang EJ (2012) Effects of ginsenoside Re on LPS-induced inflammatory mediators in BV2 microglial cells. BMC Complement Altern Med:12–196
Loane DJ, Kumar A (2016) Microglia in the TBI brain: the good, the bad, and the dysregulated. Exp Neurol 275(3):316–327
Lucas SM, Rothwell NJ, Gibson RM (2006) The role of inflammation in CNS injury and disease. Br J Pharmacol 147(Suppl 1):S232–S240
Marinova-Mutafchieva L, Sadeghian M, Broom L, Davis JB, Medhurst AD, Dexter DT (2009) Relationship between microglial activation and dopaminergic neuronal loss in the substantia nigra: a time course study in a 6-hydroxydopamine model of Parkinson’s disease. J Neurochem 8:966–975
More SV, Kumar H, Kim IS, Song SY, Choi DK (2013) Cellular and molecular mediators of neuroinflammation in the pathogenesis of Parkinson's disease. Mediat Inflamm:2013–952375
Ramachandiran S, Huang Q, Dong J, Lau SS, Monks TJ (2002) Mitogen-activated protein kinases contribute to reactive oxygen species-induced cell death in renal proximal tubule epithelial cells. Chem Res Toxicol 15(12):1635–1642
Rengarajan T, Nandakumar N, Balasubramanian MP (2012) D-Pinitol a low-molecular cyclitol prevents 7, 12-Dimethylbenz anthracene induced experimental breast cancer through regulating anti-apoptotic protein Bcl-2, mitochondrial and carbohydrate key metabolizing enzymes. Biomed Preven Nutri 2:25–30
Sethi G, Ahn KS, Sung B, Aggarwal BB (2008) Pinitol targets nuclear factor-KB activation pathway leading to inhibition of gene products associated with proliferation, apoptosis, invasion, and angiogenesis. Mol Cancer Ther 7:1604–1614
Shi H, Deng HX, Gius D, Schumacker PT, Surmeier DJ, Ma YC (2017) Sirt3 protects dopaminergic neurons from mitochondrial oxidative stress. Hum Mol Genet 26(10):1915–1926
Sivakumar S, Subramanian SP (2009) Pancreatic tissue protective nature of D-Pinitol studied in streptozotocin-mediated oxidative stress in experimental diabetic rats. Eur J Pharmacol 622(1–3):65–70
Sivakumar S, Palsamy P, Subramanian S (2010) Impact of D-pinitol on the attenuation of proinflammatory cytokines, hyperglycemia-mediated oxidative stress and protection of kidney tissue ultrastructure in streptozotocin-induced diabetic rats. Chem Biol Interact 188:237–245
Sivandzade F, Prasad S, Bhalerao A, Cucullo L (2019) NRF2 and NF-қB interplay in cerebrovascular and neurodegenerative disorders: molecular mechanisms and possible therapeutic approaches. Redox Biol:21–101059
Streeter JG (1980) Carbohydrates in soybean nodules: II. Distribution of compounds in seedlings during the onset of nitrogen fixation. Plant Physiol 66(3):471–476
Takahashi K, Rochford CDP, Neumann H (2005) Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med 201(4):647–657
Teeling JL, Perry VH (2009) Systemic infection and inflammation in acute CNS injury and chronic neurodegeneration: underlying mechanisms. Neuroscience 158:1062–1073
Ulland TK, Colonna M (2018) TREM2 - a key player in microglial biology and Alzheimer disease. Nat Rev Neurol 4(11):667–675
Von BR, Eugenínvon BL, Eugenín J (2015) Microglial cell dysregulation in brain aging and neurodegeneration. Front Aging Neurosci 7:124
World Health Organization (2012) Dementia a public health priority. [Geneva]: World Health Organization http://site.ebrary.com/lib/ucmerced/Doc?id=10718026 [accessed 14 November 2013]
Zhong L, Chen XF, Zhang ZL, Wang Z, Shi XZ, Xu K, Zhang YW, Xu H, Bu G (2015) DAP12 stabilizes the C-terminal fragment of the triggering receptor expressed on myeloid cells-2 (TREM2) and protects against LPS-induced pro-inflammatory response. J Biol Chem 290(25):15866–15877
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kong, J., Du, Z. & Dong, L. Pinitol Prevents Lipopolysaccharide (LPS)-Induced Inflammatory Responses in BV2 Microglia Mediated by TREM2. Neurotox Res 38, 96–104 (2020). https://doi.org/10.1007/s12640-020-00187-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-020-00187-z