Abstract
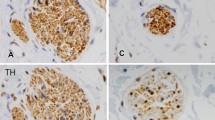
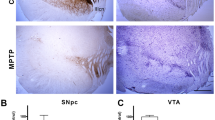
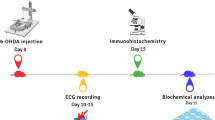
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by the accumulation of Lewy bodies and loss of dopaminergic neurons in the substantia nigra pars compacta (SNpC). MPTP is widely used to generate murine PD model. In addition to classical motor disorders, PD patients usually have non-motor symptoms related to autonomic impairment, which precedes decades before the motor dysfunction. This study’s objective is to examine the effects of MPTP on noradrenergic neurons in the hindbrain, thereby on the cardiovascular function in mice. Adult mice received 10 mg/kg/day of MPTP (4 consecutive days) to generate PD model. Systolic blood pressure was measured by tail cuff system in conscious mice, and baroreflex sensitivity was evaluated by heart rate alteration in response to a transient increase or decrease in blood pressure induced by intravenous infusion of phenylalanine (PE) or sodium nitroprusside (SNP) in anesthetized condition, respectively. Baseline heart rate and heart rate variability were analyzed in both sham and MPTP-treated mice. Dopamine, norepinephrine, and related metabolites in the plasma and brain tissues including SNpC, locus coeruleus (LC), rostroventrolateral medulla (RVLM), and nucleus tractus solitarii (NTS) were measured by liquid chromatography-mass spectrometry (LC-MS). Tyrosine hydroxylase-positive (TH+) neurons in above nuclei were quantified by immunoreactivities. We found that in addition to the loss of TH+ neurons in SNpC, MPTP treatment induced a dramatic reduction of TH+ cell counts in the LC, RVLM, and NTS. These are associated with significant decreases of dopamine, norepinephrine, and epinephrine in above nuclei. Meanwhile, MPTP induced a lasting effect of baroreflex desensitization, tachycardia, and decreased heart rate variability compared to the sham mice. Notably, MPTP treatment elevated sympathetic outflow and suppressed parasympathetic tonicity according to the heart rate power spectrum analysis. Our results indicate that the loss of TH+ neurons in the brainstem by MPTP treatment led to impaired autonomic cardiovascular function. These results suggest that MPTP treatment can be used to study the autonomic dysfunction in murine model.







Similar content being viewed by others
References
Beal MF (2001) Experimental models of Parkinson’s disease. Nat Rev Neurosci 2(5):325–334
Braak H, Rub U, Sandmann-Keil D, Gai WP, de Vos RA et al (2000) Parkinson’s disease: affection of brain stem nuclei controlling premotor and motor neurons of the somatomotor system. Acta Neuropathol 99(5):489–495
Braak H, Sastre M, Bohl JR, de Vos RA, Del Tredici K (2007) Parkinson’s disease: lesions in dorsal horn layer I, involvement of parasympathetic and sympathetic pre- and postganglionic neurons. Acta Neuropathol 113(4):421–429
Carney RM, Blumenthal JA, Stein PK, Watkins L, Catellier D, Berkman LF, Czajkowski SM, O'Connor C, Stone PH, Freedland KE (2001) Depression, heart rate variability, and acute myocardial infarction. Circulation 104(17):2024–2028
Chapleau MW, Sabharwal R (2011) Methods of assessing vagus nerve activity and reflexes. Heart Fail Rev 16(2):109–127
Chaudhuri KR, Schapira AH (2009) Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol 8(5):464–474
Dauer W, Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron 39(6):889–909
DePuy SD, Stornetta RL, Bochorishvili G, Deisseroth K, Witten I et al (2013) Glutamatergic neurotransmission between the C1 neurons and the parasympathetic preganglionic neurons of the dorsal motor nucleus of the vagus. J Neurosci 33(4):1486–1497
Espay AJ, LeWitt PA, Kaufmann H (2014) Norepinephrine deficiency in Parkinson’s disease: the case for noradrenergic enhancement. Mov Disord 29(14):1710–1719
Fox SH, Brotchie JM (2010) The MPTP-lesioned non-human primate models of Parkinson’s disease. Past, present, and future. Prog Brain Res 184:133–157
Gehrmann J, Hammer PE, Maguire CT, Wakimoto H, Triedman JK, Berul CI (2000) Phenotypic screening for heart rate variability in the mouse. Am J Physiol Heart Circ Physiol 279(2):H733–H740
Gibbons CH, Schmidt P, Biaggioni I, Frazier-Mills C, Freeman R, Isaacson S, Karabin B, Kuritzky L, Lew M, Low P, Mehdirad A, Raj SR, Vernino S, Kaufmann H (2017) The recommendations of a consensus panel for the screening, diagnosis, and treatment of neurogenic orthostatic hypotension and associated supine hypertension. J Neurol 264(8):1567–1582
Glover V, Gibb C, Sandler M (1986) The role of MAO in MPTP toxicity--a review. J Neural Transm Suppl 20:65–76
Goldstein DS, Holmes CS, Dendi R, Bruce SR, Li ST (2002) Orthostatic hypotension from sympathetic denervation in Parkinson's disease. Neurology 58(8):1247–1255
Grau CM, Greene LA (2012) Use of PC12 cells and rat superior cervical ganglion sympathetic neurons as models for neuroprotective assays relevant to Parkinson’s disease. Methods Mol Biol 846:201–211
Guyenet PG (2006) The sympathetic control of blood pressure. Nat Rev Neurosci 7(5):335–346
Guyenet PG, Stornetta RL, Bochorishvili G, Depuy SD, Burke PG et al (2013) C1 neurons: the body’s EMTs. Am J Phys Regul Integr Comp Phys 305(3):R187–R204
Jain S, Goldstein DS (2012) Cardiovascular dysautonomia in Parkinson disease: from pathophysiology to pathogenesis. Neurobiol Dis 46(3):572–580
Javitch JA, D'Amato RJ, Strittmatter SM, Snyder SH (1985) Parkinsonism-inducing neurotoxin, N-methyl-4-phenyl-1,2,3,6 -tetrahydropyridine: uptake of the metabolite N-methyl-4-phenylpyridine by dopamine neurons explains selective toxicity. Proc Natl Acad Sci U S A 82(7):2173–2177
Jin H, Zhang JR, Shen Y, Liu CF (2017) Clinical significance of REM sleep behavior disorders and other non-motor symptoms of parkinsonism. Neurosci Bull 33(5):576–584
Kalia LV, Lang AE (2015) Parkinson’s disease. Lancet 386(9996):896–912
Kaufmann H, Goldstein DS (2013) Autonomic dysfunction in Parkinson disease. Handb Clin Neurol 117:259–278
Kaufmann H, Oribe E, Miller M, Knott P, Wiltshire-Clement M, Yahr MD (1992) Hypotension-induced vasopressin release distinguishes between pure autonomic failure and multiple system atrophy with autonomic failure. Neurology 42(3 Pt 1):590–593
Kuroiwa H, Yokoyama H, Kimoto H, Kato H, Araki T (2010) Biochemical alterations of the striatum in an MPTP-treated mouse model of Parkinson’s disease. Metab Brain Dis 25(2):177–183
Lang AE, Blair RD (1984) Parkinson’s disease in 1984: an update. Can Med Assoc J 131(9):1031–1037
Li S, Le W (2017a) Biomarker discovery in Parkinson’s disease: present challenges and future opportunities. Neurosci Bull 33(5):481–482
Li S, Le W (2017b) Milestones of Parkinson’s disease research: 200 years of history and beyond. Neurosci Bull 33(5):598–602
Li Y, Shen XZ, Li L, Zhao TV, Bernstein KE, Johnson AK, Lyden P, Fang J, Shi P (2017) Brain transforming growth factor-beta resists hypertension via regulating microglial activation. Stroke 48(9):2557–2564
Loavenbruck A, Sandroni P (2015) Neurogenic orthostatic hypotension: roles of norepinephrine deficiency in its causes, its treatment, and future research directions. Curr Med Res Opin 31(11):2095–2104
Malliani A (2005) Heart rate variability: from bench to bedside. Eur J Intern Med 16(1):12–20
Mavridis M, Degryse AD, Lategan AJ, Marien MR, Colpaert FC (1991) Effects of locus coeruleus lesions on parkinsonian signs, striatal dopamine and substantia nigra cell loss after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in monkeys: a possible role for the locus coeruleus in the progression of Parkinson’s disease. Neuroscience 41(2–3):507–523
Mitsui J, Saito Y, Momose T, Shimizu J, Arai N, Shibahara J, Ugawa Y, Kanazawa I, Tsuji S, Murayama S (2006) Pathology of the sympathetic nervous system corresponding to the decreased cardiac uptake in 123I-metaiodobenzylguanidine (MIBG) scintigraphy in a patient with Parkinson disease. J Neurol Sci 243(1–2):101–104
Mitsumoto Y, Mori A (2018) Acute restraint stress augments 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity via increased toxin uptake into the brain in C57BL/6 mice. Neurosci Bull 34(5):849-853. https://doi.org/10.1007/s12264-018-0254-2
Nicklas WJ, Vyas I, Heikkila RE (1985) Inhibition of NADH-linked oxidation in brain mitochondria by 1-methyl-4-phenyl-pyridine, a metabolite of the neurotoxin, 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. Life Sci 36(26):2503–2508
Niimi Y, Ieda T, Hirayama M, Koike Y, Sobue G, Hasegawa Y, Takahashi A (1999) Clinical and physiological characteristics of autonomic failure with Parkinson’s disease. Clin Auton Res 9(3):139–144
Pavlov VA, Tracey KJ (2015) Neural circuitry and immunity. Immunol Res 63(1–3):38–57
Reardon M, Malik M (1996) Changes in heart rate variability with age. Pacing Clin Electrophysiol 19(11 Pt 2):1863–1866
Rekha KR, Selvakumar GP, Santha K, Inmozhi Sivakamasundari R (2013) Geraniol attenuates alpha-synuclein expression and neuromuscular impairment through increase dopamine content in MPTP intoxicated mice by dose dependent manner. Biochem Biophys Res Commun 440(4):664–670
Roy S, Jaryal AK, Srivastava AK, Deepak KK (2016) Cardiovagal baroreflex sensitivity in Parkinson’s disease and multiple-system atrophy. J Clin Neurol 12(2):218–223
Russell SM, Davey J, Mayer RJ (1979) The vectorial orientation of human monoamine oxidase in the mitochondrial outer membrane. Biochem J 181(1):7–14
Samuels ER, Szabadi E (2008) Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic function part II: physiological and pharmacological manipulations and pathological alterations of locus coeruleus activity in humans. Curr Neuropharmacol 6(3):254–285
Schreihofer AM, Guyenet PG (2002) The baroreflex and beyond: control of sympathetic vasomotor tone by GABAergic neurons in the ventrolateral medulla. Clin Exp Pharmacol Physiol 29(5–6):514–521
Senard JM, Valet P, Durrieu G, Berlan M, Tran MA, Montastruc JL, Rascol A, Montastruc P (1990) Adrenergic supersensitivity in parkinsonians with orthostatic hypotension. Eur J Clin Investig 20(6):613–619
Senard JM, Rascol O, Durrieu G, Tran MA, Berlan M, Rascol A, Montastruc JL (1993) Effects of yohimbine on plasma catecholamine levels in orthostatic hypotension related to Parkinson disease or multiple system atrophy. Clin Neuropharmacol 16(1):70–76
Shen XZ, Li Y, Li L, Shah KH, Bernstein KE, Lyden P, Shi P (2015) Microglia participate in neurogenic regulation of hypertension. Hypertension 66(2):309–316
Shibao CA, Kaufmann H (2017) Pharmacotherapy of cardiovascular autonomic dysfunction in Parkinson disease. CNS Drugs 31(11):975–989
Siaud P, Puech R, Assenmacher I, Alonso G (1990) Adrenergic innervation of the dorsal vagal motor nucleus: possible involvement in inhibitory control of gastric acid and pancreatic insulin secretion. Cell Tissue Res 259(3):535–542
Srinivasan J, Schmidt WJ (2003) Potentiation of parkinsonian symptoms by depletion of locus coeruleus noradrenaline in 6-hydroxydopamine-induced partial degeneration of substantia nigra in rats. Eur J Neurosci 17(12):2586–2592
Szot P, Knight L, Franklin A, Sikkema C, Foster S, Wilkinson CW, White SS, Raskind MA (2012) Lesioning noradrenergic neurons of the locus coeruleus in C57Bl/6 mice with unilateral 6-hydroxydopamine injection, to assess molecular, electrophysiological and biochemical changes in noradrenergic signaling. Neuroscience 216:143–157
Thireau J, Zhang BL, Poisson D, Babuty D (2008) Heart rate variability in mice: a theoretical and practical guide. Exp Physiol 93(1):83–94
Umehara T, Oka H, Nakahara A, Matsuno H, Toyoda C (2018) High norepinephrinergic orthostatic hypotension in early Parkinson’s disease. Parkinsonism Relat Disord 55:97–102
Watanabe Y, Himeda T, Araki T (2005) Mechanisms of MPTP toxicity and their implications for therapy of Parkinson’s disease. Med Sci Monit 11(1):Ra17–Ra23
Weinshenker D (2018) Long road to ruin: noradrenergic dysfunction in neurodegenerative disease. Trends Neurosci 41(4):211–223
Williams TD, Lightman SL, Bannister R (1985) Vasopressin secretion in progressive autonomic failure: evidence for defective afferent cardiovascular pathways. J Neurol Neurosurg Psychiatry 48(3):225–228
Xue B, Skala K, Jones TA, Hay M (2004) Diminished baroreflex control of heart rate responses in otoconia-deficient C57BL/6JEi head tilt mice. Am J Physiol Heart Circ Physiol 287(2):H741–H747
Funding
This work was supported by research foundations of the National Natural Science Foundation of China 31771266 and 81700365 to P.S., 81670378 to X.Z.S., 31771266 to G.X.M., and Zhejiang Province 2014KYB006, 2015C33135, 2013ZA009 to X.L.L.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Peng Shi is the Principal Investigator
Rights and permissions
About this article
Cite this article
Liu, X., Wei, B., Bi, Q. et al. MPTP-Induced Impairment of Cardiovascular Function. Neurotox Res 38, 27–37 (2020). https://doi.org/10.1007/s12640-020-00182-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-020-00182-4