Abstract
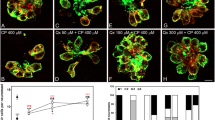
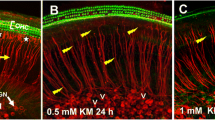
A wide variety of ototoxic drugs are capable of damaging the sensory hair cells in the mammalian cochlea resulting in permanent hearing loss. However, the toxic properties of these drugs suggest that some could potentially damage cochlear support cells as well. To test the hypothesis, we treated postnatal day three rat cochlear cultures with toxic doses of gentamicin, cisplatin, mefloquine, and cadmium. Gentamicin primarily destroyed the hair cells and disrupted the intercellular connection with the surrounding support cells. Gentamicin-induced hair cell death was initiated through the caspase-9 intrinsic apoptotic pathway followed by activation of downstream executioner caspase-3. In contrast, cisplatin, mefloquine, and cadmium initially damaged the support cells and only later damaged the hair cells. Support cell death was initiated through the caspase-8 extrinsic apoptotic pathway followed later by downstream activation of caspase-3. Cisplatin, mefloquine, and cadmium significantly reduced the expression of actin and laminin, in the extracellular matrix, leading to significant disarray of the sensory epithelium.




Similar content being viewed by others
Abbreviations
- Cd:
-
Cadmium
- CIS:
-
Cisplatin
- DC:
-
Deiters cell
- ECM:
-
Extracellular matrix
- GM:
-
Gentamycin
- HC:
-
Hensen cells
- IHC:
-
Inner hair cells
- IS:
-
Inner sulcus
- ISC:
-
Inner sulcus cells
- MEF:
-
Mefloquine
- OHC:
-
Outer hair cells
- OS:
-
Outer sulcus
- PC:
-
Pillar cell
- PBS:
-
Phosphate buffered saline
- SC:
-
Support cells
References
Alam SA, Ikeda K, Oshima T, Suzuki M, Kawase T, Kikuchi T, Takasaka T (2000) Cisplatin-induced apoptotic cell death in Mongolian gerbil cochlea. Hear Res 141:28–38
Anttonen T, Belevich I, Kirjavainen A, Laos M, Brakebusch C, Jokitalo E, Pirvola U (2014) How to bury the dead: elimination of apoptotic hair cells from the hearing organ of the mouse. J Assoc Res Otolaryngol 15:975–992
Boudreau N, Sympson CJ, Werb Z, Bissell MJ (1995) Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science 267:891–893
Bozzo C, Sabbatini M, Tiberio R, Piffanelli V, Santoro C, Cannas M (2006) Activation of caspase-8 triggers anoikis in human neuroblastoma cells. Neurosci Res 56:145–153
Chiarugi P, Giannoni E (2008) Anoikis: a necessary death program for anchorage-dependent cells. Biochem Pharmacol 76:1352–1364
Chuang CY, Degendorfer G, Davies MJ (2014) Oxidation and modification of extracellular matrix and its role in disease. Free Radic Res 48:970–989
Chun J, Joo EJ, Kang M, Kim YS (2013) Platycodin D induces anoikis and caspase-mediated apoptosis via p38 MAPK in AGS human gastric cancer cells. J Cell Biochem 114:456–470
Clerici WJ, Hensley K, DiMartino DL, Butterfield DA (1996) Direct detection of ototoxicant-induced reactive oxygen species generation in cochlear explants. Hear Res 98:116–124
Collado MS, Burns JC, Meyers JR, Corwin JT (2011) Variations in shape-sensitive restriction points mirror differences in the regeneration capacities of avian and mammalian ears. PLoS One 6:e23861
D’Arcangelo G, Miao GG, Chen S-C, Soares HD, Morgan JI, Curran T (1975) A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature 374:719–723
D’Arcy MS (2019) Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int 43:582–592
Davies D (2011) Cell-extracellular matrix versus cell-cell interactions during the development of the cochlear-vestibular ganglion. J Neurosci Res 89:1375–1387
Deng L, Ding D, Su J, Manohar S, Salvi R (2013) Salicylate selectively kills cochlear spiral ganglion neurons by paradoxically up-regulating superoxide. Neurotox Res 24:307–319
Ding D, Salvi R (2010) Paraquat-induced damage to the cochlear hair cells. Dalian Ding Science of the Inner Ear;China Science and Technology Press 325–9
Ding D, Jiang H, Wang P, Salvi R (2007) Cell death after co-administration of cisplatin and ethacrynic acid. Hear Res 226:129–139
Ding D, He J, Allman BL, Yu D, Jiang H, Seigel GM et al (2011) Cisplatin ototoxicity in rat cochlear organotypic cultures. Hear Res 282:196–203
Ding D, Qi W, Yu D, Jiang H, Han C, Katsuno K et al (2013a) NAD+ prevents mefloquine-induced neuroaxonal and hair cell degeneration through reduction of caspase-3-mediated apoptosis. PLoS One
Ding D, Qi W, Yu D, Jiang H, Han C, Kim MJ et al (2013b) Addition of exogenous NAD+ prevents mefloquine-induced neuroaxonal and hair cell degeneration through reduction of caspase-3-mediated apoptosis in cochlear organotypic cultures. PLoS One 8:e79817
Dong Y, Ding D, Jiang H, Shi JR, Salvi R, Roth JA (2014) Ototoxicity of paclitaxel in rat cochlear organotypic cultures. Toxicol Appl Pharmacol 280:526–533
Eichler T, Ma Q, Kelly C, Mishra J, Parikh S, Ransom RF, Devarajan P, Smoyer WE (2006) Single and combination toxic metal exposures induce apoptosis in cultured murine podocytes exclusively via the extrinsic caspase 8 pathway. Toxicol Sci 90:392–399
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516
Frisch SM (2000) Anoikis. Methods Enzymol 322:472–479
Frisch SM, Screaton RA (2001) Anoikis mechanisms. Curr Opin Cell Biol 13:555–562
Gonzalez-Garcia JA, Nevado J, Garcia-Berrocal JR, Sanchez-Rodriguez C, Trinidad A, Sanz R et al (2010) Endogenous protection against oxidative stress caused by cisplatin: role of superoxide dismutase. Acta Otolaryngol 130:453–457
Hamill KJ, Kligys K, Hopkinson SB, Jones JC (2009) Laminin deposition in the extracellular matrix: a complex picture emerges. J Cell Sci 122:4409–4417
Hammar E, Parnaud G, Bosco D, Perriraz N, Maedler K, Donath M, Rouiller DG, Halban PA (2004) Extracellular matrix protects pancreatic beta-cells against apoptosis: role of short- and long-term signaling pathways. Diabetes 53:2034–2041
He Z, Guo L, Shu Y, Fang Q, Zhou H, Liu Y, Liu D, Lu L, Zhang X, Ding X, Liu D, Tang M, Kong W, Sha S, Li H, Gao X, Chai R (2017) Autophagy protects auditory hair cells against neomycin-induced damage. Autophagy 13:1884–1904
Heaney DL, Schulte BA, Niedzielski AS (2002) Dystroglycan expression in the developing and senescent gerbil cochlea. Hear Res 174:9–18
Hordichok AJ, Steyger PS (2007) Closure of supporting cell scar formations requires dynamic actin mechanisms. Hear Res 232:1–19
Hou S, Chen J, Yang J (2019) Autophagy precedes apoptosis during degeneration of the Kolliker’s organ in the development of rat cochlea. Eur J Histochem 63
Huang HL, Fang LW, Lu SP, Chou CK, Luh TY, Lai MZ (2003) DNA-damaging reagents induce apoptosis through reactive oxygen species-dependent Fas aggregation. Oncogene 22:8168–8177
Huang CR, Jin ZX, Dong L, Tong XP, Yue S, Kawanami T, Sawaki T, Sakai T, Miki M, Iwao H, Nakajima A, Masaki Y, Fukushima Y, Tanaka M, Fujita Y, Nakajima H, Okazaki T, Umehara H (2010) Cisplatin augments FAS-mediated apoptosis through lipid rafts. Anticancer Res 30:2065–2071
Hultcrantz M, Li HS (1995) Degeneration patterns of actin distribution in the organ of corti in two genotypes of mice. ORL: J Oto-Rhino-Laryngol Relat Spec 57:1–4
Ishii K, Schroter-Kermani C, Xu D, Merker HJ, Jahnke V (1992) Extracellular matrix in the rat spiral limbus. Eur Arch Otorhinolaryngol 249:224–230
Kerr JF, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26:239–257
Kim SJ, Jeong HJ, Myung NY, Kim MC, Lee JH, So HS, Park RK, Kim HM, Um JY, Hong SH (2008) The protective mechanism of antioxidants in cadmium-induced ototoxicity in vitro and in vivo. Environ Health Perspect 116:854–862
Menardo J, Tang Y, Ladrech S, Lenoir M, Casas F, Michel C et al (2012) Oxidative stress, inflammation, and autophagic stress as the key mechanisms of premature age-related hearing loss in SAMP8 mouse cochlea. Antioxid Redox Signal 16:263–274
Micheau O, Solary E, Hammann A, Dimanche-Boitrel MT (1999) Fas ligand-independent, FADD-mediated activation of the Fas death pathway by anticancer drugs. J Biol Chem 274:7987–7992
Milner R, Campbell IL (2003) The extracellular matrix and cytokines regulate microglial integrin expression and activation. J Immunol 170:3850–3858
Miyazaki T, Shen M, Fujikura D, Tosa N, Kim HR, Kon S et al (2004) Functional role of death-associated protein 3 (DAP3) in anoikis. J Biol Chem 279:44667–44672
Nakagawa T, Yamane H, Takayama M, Sunami K, Nakai Y (1998) Apoptosis of guinea pig cochlear hair cells following chronic aminoglycoside treatment. Eur Arch Otorhinolaryngol 255:127–131
Nicotera T, Henderson D, Zheng X-Y, Ding D, McFadden SL (1999) Reactive oxygen species, apoptosis and necrosis in noise-exposed cochleas of chinchillas. Abstr Assoc Res Otolaryngol 22:626
Pietrogrande G, Mabotuwana N, Zhao Z, Abdolhoseini M, Johnson SJ, Nilsson M et al (2018) Chronic stress induced disturbances in laminin: a significant contributor to modulating microglial pro-inflammatory tone? Brain Behav Immun 68:23–33
Qi W, Ding D, Salvi RJ (2008) Cytotoxic effects of dimethyl sulphoxide (DMSO) on cochlear organotypic cultures. Hear Res 236:52–60
Racz E, Gaal B, Kecskes S, Matesz C (2014) Molecular composition of extracellular matrix in the vestibular nuclei of the rat. Brain Struct Funct 219:1385–1403
Raphael Y (2002) Cochlear pathology, sensory cell death and regeneration. Br Med Bull 63:25–38
Raphael Y, Altschuler RA (1991) Reorganization of cytoskeletal and junctional proteins during cochlear hair cell degeneration. Cell Motil Cytoskeleton 18:215–227
Raphael Y, Altschuler RA (2003) Structure and innervation of the cochlea. Brain Res Bull 60:397–422
Richardson GP, Crossin KL, Chuong CM, Edelman GM (1987) Expression of cell adhesion molecules during embryonic induction. III. Development of the otic placode. Dev Biol 119:217–230
Setz C, Brand Y, Radojevic V, Hanusek C, Mullen PJ, Levano S, Listyo A, Bodmer D (2011) Matrix metalloproteinases 2 and 9 in the cochlea: expression and activity after aminoglycoside exposition. Neuroscience 181:28–39
Shanmugathasan M, Jothy S (2000) Apoptosis, anoikis and their relevance to the pathobiology of colon cancer. Pathol Int 50:273–279
Stupack DG, Puente XS, Boutsaboualoy S, Storgard CM, Cheresh DA (2001) Apoptosis of adherent cells by recruitment of caspase-8 to unligated integrins. J Cell Biol 155:459–470
Taddei ML, Giannoni E, Fiaschi T, Chiarugi P (2012) Anoikis: an emerging hallmark in health and diseases. J Pathol 226:380–393
Tsuprun V, Santi P (1999) Ultrastructure and immunohistochemical identification of the extracellular matrix of the chinchilla cochlea. Hear Res 129:35–49
Yu D, Ding D, Jiang H, Stolzberg D, Salvi R (2011) Mefloquine damage vestibular hair cells in organotypic cultures. Neurotox Res 20:51–58
Yu J, Ding D, Sun H, Salvi R, Roth J (2015) Neurotoxicity of trimethyltin in rat cochlear organotypic cultures. Neurotox Res 28:43–54
Zhang J, Sun H, Salvi R, Ding D (2018) Paraquat initially damages cochlear support cells leading to anoikis-like hair cell death. Hear Res 364:129–141
Funding
The research is supported in part by a grant from NIOSH (R01OH010235) and in part by the Foundation of Science and Technology Commission of Shanghai Municipality (#15140900900).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ding, D., Zhang, J., Jiang, H. et al. Some Ototoxic Drugs Destroy Cochlear Support Cells Before Damaging Sensory Hair Cells. Neurotox Res 37, 743–752 (2020). https://doi.org/10.1007/s12640-020-00170-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-020-00170-8