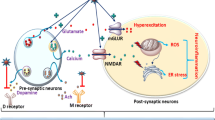
Abstract
Following anoxia, a rapid and marked mitochondrial-linked cell death occurs in the cerebral cortex of newborn rats which leads to insult advancement within a couple of days and causes lifelong neurobehavioral abnormalities. The present study investigated the role of 2,4 dinitrophenol (2,4 DNP) in three doses, i.e.,1, 2.5, and 5 mg/kg on anoxia-induced time-dependent mitochondrial dysfunction and associated neurobehavioral outcome using a well-established global model of anoxia. Briefly, rat pups of 30-h age (P2) were subjected to two episodes of anoxia (10 min each) at 24 h of the time interval in an enclosed chamber supplied with 100% N2 and immersed in a water bath (35–37 °C) to avoid hypothermia. Results demonstrated that the uncoupler 2,4 DNP, in the dose 2.5 and 5 mg/kg injected i.p. within 5 min after second anoxic episode significantly (P < 0.05) preserved mitochondrial function on day 7 preferentially by maintaining mitochondrial membrane potential (MMP) and inhibiting mitochondrial permeability transition (MPT) pore. Further, 2,4 DNP preserved mitochondrial function by improving different states of mitochondrial respiration (s2, s3, s4, s5), respiratory control ratio (RCR), antioxidant enzyme system like superoxide dismutase (SOD) and catalase (CAT), and mitochondrial complex enzymes (I, II, IV, V) after anoxia. Furthermore, a marked decrease in the levels of expression of cytochrome C (cyt C) and pro-apoptotic (Bcl-2 family) and apoptotic (caspase-9/3) proteins was observed on day 7 indicating that the treatment with 2,4 DNP prevented mitochondrial dysfunction and further insult progression (day 1 to day 7). Moreover, 2,4 DNP decreased the apoptotic cell death on day 7 and overall improved the neurobehavioral outcomes like reflex latency and hanging latency which suggests its role in treating neonatal anoxia.











Similar content being viewed by others
Abbreviations
- 2,4 DNP:
-
2,4 Dinitrophenol
- NO:
-
Nitric oxide
- UPCs:
-
Mitochondrial uncouplers
- MMP`:
-
Mitochondrial membrane potential
- MPT:
-
Mitochondrial permeability transition
- RCR:
-
Respiratory control ratio
- ETC:
-
Electron transport chain
- LPO:
-
Lipid peroxidation
- SOD:
-
Superoxide dismutase
- CAT:
-
Catalase
- Cyt C:
-
Cytochrome C
- GABA:
-
Gamma-aminobutyric acid
- CNS:
-
Central nervous system
- EAA:
-
Excitatory amino acid
- ATP:
-
Adenosine triphosphate
- ADP:
-
Adenosine diphosphate
- NADH:
-
Nicotinamide adenine dinucleotide
- N2 :
-
Nitrogen
- ROS:
-
Reactive oxygen species
- RNS:
-
Reactive nitrogen species
- Bcl-2:
-
B cell lymphoma 2
- Bax:
-
Bcl-2-associated X protein
- Ca2+ :
-
Calcium ion
- s:
-
State
- P:
-
Pyruvate
- M:
-
Malate
- i.p.:
-
Intraperitoneal
- TMRM:
-
Tetramethylrhodamine methyl ester
- HEPES:
-
(4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid)
- EGTA:
-
(Ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid)
- DMSO:
-
Dimethyl sulfoxide
- SDH:
-
Succinate dehydrogenase
- SDS-PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- PVDF:
-
Polyvinylidene fluoride
- FITC:
-
Fluorescein isothiocyanate
- PBS:
-
Phosphate buffer saline
- ECL:
-
Chemiluminescence
- PI:
-
Propidium iodide
- ANT:
-
Adenosine nucleotide translocase
- ER:
-
Endoplasmic reticulum
- MAMs:
-
Mitochondria-associated ER membranes
- NBT:
-
Nitro blue tetrazolium
- dfz:
-
Diformazan
- MDA:
-
Malondialdehyde
- ANOVA:
-
Analysis of variance
References
Beers RF, Sizer IW (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195(1):133–140
Bel FV, Groenendaal F (2016) Drugs for neuroprotection after birth asphyxia: pharmacologic adjuncts to hypothermia. Semin Perinatol 40(3):152–159. https://doi.org/10.1053/j.semperi.2015.12.003
Berman SB, Hastings TG (1999) Dopamine oxidation alters mitochondrial respiration and induces permeability transition in brain mitochondria. J Neurochem 73(3):1127–1137
Boardman WW (1935) Rapidly developing cataracts after dinitrophenol. Calif West Med 43(2):118–119
Borutaite V, Toleikis A, Brown GC (2013) In the eye of the storm: mitochondrial damage during heart and brain ischaemia. FEBS J 280:4999–5014
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1-2):248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Cerio FGD, Lara-Celador I, Alvarez A, Hilario E (2013) Neuroprotective therapies after perinatal hypoxic-ischemic brain injury. Brain Sci 3:191–214
Cheng G, Kong RH, Zhang LM, Zhang JN (2012) Mitochondria in traumatic brain injury and mitochondrial-targeted multipotential therapeutic strategies. Br J Pharmacol 167(4):699–719. https://doi.org/10.1111/j.1476-5381.2012.02025.x
Chowdhury I, Tharakan B, Bhat GK (2008) Caspases—an update. Comparative Biochemistry and Physiology Part B: Biochem Mol Biol 151:10–27
Diano S, Matthews RT, Patrylo P, Yang L, Beal MF, Barnstable CJ, Horvath TL (2003) Uncoupling protein 2 prevents neuronal death including that occurring during seizures: a mechanism for preconditioning. Endocrinol 144(11):5014–5021. https://doi.org/10.1210/en.2003-0667
Ennis SR, Keep RF (2006) Effects of 2,4-dinitrophenol on ischemia-induced blood-brain barrier disruption. Acta Neurochir Suppl 96:295–298. https://doi.org/10.1007/3-211-30714-1_63
Erpapazoglou Z, Mouton-Liger F, Corti O (2017) From dysfunctional endoplasmic reticulum-mitochondria coupling to neurodegeneration. Neurochem Int 17:30081–30085. https://doi.org/10.1016/j.neuint.2017.03.021.
Fan L-W, Lin S, Pang Y, Lei M, Zhang F, Rhodes PG, Cai Z (2005) Hypoxia-ischemia induced neurological dysfunction and brain injury in the neonatal rat. Behav Brain Res 165(1):80–90. https://doi.org/10.1016/j.bbr.2005.06.033
Feng J, Lu S, Ding Y, Zheng M, Wang X (2016) Homocysteine activates T cells by enhancing endoplasmic reticulum-mitochondria coupling and increasing mitochondrial respiration. Protein Cell 7(6):391–402. https://doi.org/10.1007/s13238-016-0245-x
Fiske CH, Subbarow Y (1925) The colorimetric determination of phosphorus. J biol Chem 66:375–400
Forkink M, Manjeri GR, Liemburg-Apers DC, Nibbeling E, Blanchard M, Wojtala A, Smeitink JA, Wieckowski MR, Willems PH, Koopman WJ (2014) Mitochondrial hyperpolarization during chronic complex I inhibition is sustained by low activity of complex II, III, IV and V. Biochim Biophys Acta 1837(8):1247–1256. https://doi.org/10.1016/j.bbabio.2014.04.008
Gilmer LK, Ansari MA, Roberts KN, Scheff SW (2010) Age-related changes in mitochondrial respiration and oxidative damage in the cerebral cortex of the Fischer 344 rat. Mech Ageing Dev 131(2):133–143. https://doi.org/10.1016/j.mad.2009.12.011
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem 126(1):131–138. https://doi.org/10.1016/0003-2697(82)90118-X
Griffiths DE, Houghton RL (1974) Studies on energy-linked reactions: modified mitochondrial ATPase of oligomycin-resistant mutants of Saccharomyces cerevisiae. Eur J Biochem/ FEBS 46(1):157–167. https://doi.org/10.1111/j.1432-1033.1974.tb03608.x
Grundlingh J, Dargan PI, El-Zanfaly M, Wood DM (2011) 2,4-Dinitrophenol (DNP): a weight loss agent with significant acute toxicity and risk of death. J Med Toxicol 7(3):205–212. https://doi.org/10.1007/s13181-011-0162-6
Harper JA, Dickinson K, Brand MD (2001) Mitochondrial uncoupling as a target for drug development for the treatment of obesity. Obes Rev 2:255–265
Hermans RHM, Hunter DE, McGivern RF, Cain CD, Longo LD (1992) Behavioral sequelae in young rats of acute intermittent antenatal hypoxia. Neurotoxicol Teratol 14(2):119–129. https://doi.org/10.1016/0892-0362(92)90060-N
Huang SG (2002) Development of a high throughput screening assay for mitochondrial membrane potential in living cells. J Biomol Screen 7(4):383–389. https://doi.org/10.1177/108705710200700411
Kakkar P, Das B, Viswanathan PN (1984) A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys 21:130–132
Kenwood BM, Weaver JL, Bajwa A, Poon IK, Byrne FL, Murrow BA, Calderone JA, Huang L, Divakaruni AS, Tomsig JL, Okabe K, Lo RH, Cameron Coleman G, Columbus L, Yan Z, Saucerman JJ, Smith JS, Holmes JW, Lynch KR, Ravichandran KS, Uchiyama S, Santos WL, Rogers GW, Okusa MD, Bayliss DA, Hoehn KL (2014) Identification of a novel mitochondrial uncoupler that does not depolarize the plasma membrane. Mol Metabol 3(2):114–123. https://doi.org/10.1016/j.molmet.2013.11.005
Koch RA, Lee RC, Tainter ML (1935) Dinitrophenol on liver function. Calif West Med 43(5):337–339
Korde AS, Pettigrew LC, Craddock SD, Maragos WF (2005) The mitochondrial uncoupler 2,4-dinitrophenol attenuates tissue damage and improves mitochondrial homeostasis following transient focal cerebral ischemia. J Neurochem 94(6):1676–1684. https://doi.org/10.1111/j.1471-4159.2005.03328.x
Liu D, Chan SL, de Souza-Pinto NC, Slevin JR, Wersto RP, Zhan M, Mustafa K, de Cabo R, Mattson MP (2006) Mitochondrial UCP4 mediates an adaptive shift in energy metabolism and increases the resistance of neurons to metabolic and oxidative stress. NeuroMolecular Med 8(3):389–413. https://doi.org/10.1385/NMM:8:3:389
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Lozinsky OV, Lushchak OV, Storey JM, Storey KB, Lushchak VI (2013) The mitochondrial uncoupler 2,4-dinitrophenol attenuates sodium nitroprusside-induced toxicity in Drosophila melanogaster: potential involvement of free radicals. Comp Biochem Physiol C Toxicol Pharmacol 158(4):244–252. https://doi.org/10.1016/j.cbpc.2013.09.002
Mehta SL, Manhas N, Raghubir R (2007) Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev 54:34–66
Old SL, Johnson MA (1989) Methods of microphotometric assay of succinate dehydrogenase and cytochrome c oxidase activities for use on human skeletal muscle. Histochem J 21(9-10):545–555. https://doi.org/10.1007/BF01753355
Pandya JD, Pauly JR, Nukala VN, Sebastian AH, Day KM, Korde AS, Maragos WF, Hall ED, Sullivan PG (2007) Post-injury administration of mitochondrial uncouplers increases tissue sparing and improves behavioral outcome following traumatic brain injury in rodents. J Neurotrauma 24(5):798–811. https://doi.org/10.1089/neu.2006.3673
Patel SP, Sullivan PG, Pandya JD, Rabchevsky AG (2009) Differential effects of the mitochondrial uncoupling agent, 2,4-dinitrophenol, or the nitroxide antioxidant, Tempol, on synaptic or nonsynaptic mitochondria after spinal cord injury. J Neurosci Res 87(1):130–140. https://doi.org/10.1002/jnr.21814
Perez-Pinzon MA, Xu GP, Born J, Lorenzo J, Busto R, Rosenthal M, Sick TJ (1999) Cytochrome C is released from mitochondria into the cytosol after cerebral anoxia or ischemia. J Cereb Blood Flow Metab 19(1):39–43. https://doi.org/10.1097/00004647-199901000-00004
Petrenko AY, Cherkashina DV, Somov AY, Tkacheva EN, Semenchenko OA, Lebedinsky AS, Fuller BJ (2010) Reversible mitochondrial uncoupling in the cold phase during liver preservation/reperfusion reduces oxidative injury in the rat model. Cryobiology 60(3):293–300. https://doi.org/10.1016/j.cryobiol.2010.02.001
Pizzo P, Pozzan T (2007) Mitochondria–endoplasmic reticulum choreography: structure and signaling dynamics. Trends Cell Biol 17(10):511–517. https://doi.org/10.1016/j.tcb.2007.07.011
Puka-Sundvall M, Wallin C, Gilland E, Hallin U, Wang X, Sandberg M, Karlsson J, Blomgren K, Hagberg H (2000) Impairment of mitochondrial respiration after cerebral hypoxia–ischemia in immature rats: relationship to activation of caspase-3 and neuronal injury. Dev Brain Res 125:43–50
Rice JE III, Vannucci RC, Brierley JB (1981) The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol 9(2):131–141. https://doi.org/10.1002/ana.410090206
Rosenberg AA, Parks JK, Murdaugh E, Parker WD Jr (1989) Mitochondrial function after asphyxia in newborn lambs. Stroke 20(5):674–679. https://doi.org/10.1161/01.STR.20.5.674
Samaiya PK, Krishnamurthy S (2015) Characterization of mitochondrial bioenergetics in neonatal anoxic model of rats. J Bioenerg Biomembr 47(3):217–222. https://doi.org/10.1007/s10863-015-9603-2
Samaiya PK, Narayan G, Kumar A, Krishnamurthy S (2016) Neonatal anoxia leads to time dependent progression of mitochondrial linked apoptosis in rat cortex and associated long term sensorimotor deficits. Int J Dev Neurosci 52:55–65. https://doi.org/10.1016/j.ijdevneu.2016.05.005
Schlagowski AI, Singh F, Charles AL, Gali Ramamoorthy T, Favret F, Piquard F, Geny B, Zoll J (2014) Mitochondrial uncoupling reduces exercise capacity despite several skeletal muscle metabolic adaptations. J Appl Physiol (1985) 116:364–375
Shapiro BL, Feigal RJ, Lam LF (1979) Mitrochondrial NADH dehydrogenase in cystic fibrosis. Proc Natl Acad Sci U S A 76(6):2979–2983. https://doi.org/10.1073/pnas.76.6.2979
Storrie B, Amadden E (1990) Isolation of subcellular organelles. Methods Enzymol 182:203–225. https://doi.org/10.1016/0076-6879(90)82018-W
Sunderman F, Marzouk A, Hopfer S, Zaharia O, Reid M (1985) Increased lipid peroxidation in tissues of nickel chloride-treated rats. Ann Clin Lab Sci 15:229–236
Tainter ML, Cutting WC, Stockton AB (1934) Use of dinitrophenol in nutritional disorders: a critical survey of clinical results. Am J Public Health Nations Health 24:1045–1053
Tedeschi H, Harris DL (1958) Some observations on the photometric estimation of mitochondrial volume. Biochim Biophys Acta 28(2):392–402. https://doi.org/10.1016/0006-3002(58)90487-6
Utkina-Sosunova IV, Niatsetskaya ZV, Sosunov SA, Ratner VI, Matsiukevich D, Ten VS (2013) Nelfinavir inhibits intra-mitochondrial calcium influx and protects brain against hypoxic-ischemic injury in neonatal mice. PLoS One 8(4):e62448. https://doi.org/10.1371/journal.pone.0062448
Wassink G, Gunn ER, Drury PP, Bennet L, Gunn AJ (2014) The mechanisms and treatment of asphyxial encephalopathy. Front Neurosci 8:40
Zhang Y, Marcillat O, Giulivi C, Ernster L, Davies KJ (1990) The oxidative inactivation of mitochondrial electron transport chain components and ATPase. J Biol Chem 265:16330–16336
Funding
SK is thankful to Department of Biotechnology (DBT), New Delhi, India, for assistance in terms of research grant [102/IFD/SAN/4654/2011-2012].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The experimental procedures were approved by the Institutional Animal Ethical Committee of BHU (Protocol No. Dean/11-12/CAEC/328). All experiments were performed as per guidelines of laboratory animal care (National Research Council US Committee for the Update of the Guide for the Care and Use of Laboratory Animals 2011) guidelines.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Samaiya, P.K., Narayan, G., Kumar, A. et al. 2,4 Dinitrophenol Attenuates Mitochondrial Dysfunction and Improves Neurobehavioral Outcomes Postanoxia in Neonatal Rats. Neurotox Res 34, 121–136 (2018). https://doi.org/10.1007/s12640-018-9873-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-018-9873-7