Abstract
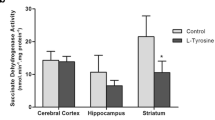
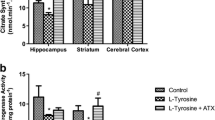
Tyrosinemia is a rare disease caused by a single mutation to the gene that code for the enzyme responsible for tyrosine catabolism. Because the mechanisms underlying the neurological dysfunction in hypertyrosinemic patients are poorly understood, we evaluated the in vitro and in vivo effect of l-tyrosine on the activities of the enzymes citrate synthase, malate dehydrogenase, succinate dehydrogenase and complexes of the mitochondrial respiratory chain in the brains and livers of young rats. Thirty-day-old Wistar rats were killed by decapitation, and the brains and livers were harvested. l-Tyrosine (0.1, 1.0, 2.0 or 4.0 mM) was added to the reaction medium. For in vivo studies, Wistar rats were killed 1 h after a single intraperitoneal injection of either tyrosine (500 mg/kg) or saline. The activities of energy metabolism enzymes were evaluated. In this research, we demonstrated in vitro that l-tyrosine inhibited citrate synthase activity in the posterior cortex and that succinate dehydrogenase was increased in the posterior cortex, hippocampus, striatum and liver. The complex I activity was only inhibited in the hippocampus, whereas complex II activity was inhibited in the hippocampus, cortex and liver. Complex IV activity decreased in the posterior cortex. The acute administration of l-tyrosine inhibited enzyme malate dehydrogenase, citrate synthase and complexes II, II–III and IV in the posterior cortex and liver. The enzyme succinate dehydrogenase and complex I activity were inhibited in the posterior cortex and increased in the striatum. These results suggest impairment in energy metabolism that is likely mediated by oxidative stress.








Similar content being viewed by others
References
Adam-Vizi V (2005) Production of reactive oxygen species in brain mitochondria: contribution by electron transport chain and non-electron transport chain sources. Antioxid Redox Signal 7:1140–1149
Boekema EJ, Braun HP (2007) Supramolecular structure of the mitochondrial oxidative phosphorylation system. J Chem Biol 28:1–4
Bongiovanni R, Yamamoto BK, Simpson C, Jaskiw GE (2003) Pharmacokinetics of systemically administered tyrosine: a comparison of serum, brain tissue and in vivo microdialysate levels in the rat. J Neurochem 87:310–317
Brennan WA, Bird ED, Aprille JR (1985) Regional mitochondrial respiratory activity in Huntington’s disease brain. J Neurochem 44:1948–1950
Cadenas E, Davies KJA (2000) Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med 29:222–230
Carbonell T, Rama R (2007) Oxidative stress and early neurological deterioration in ischemic stroke. Curr Med Chem 14:857–874
Cassina A, Radi R (1996) Differential inhibitory Action of nitric oxide and peroxynitrite on mitochondrial electron transport. Arch Biochem Biophys 328:309–316
Corrêa C, Amboni G, Assis LC, Martins MR, Kapczinski F, Streck EL, Quevedo J (2007) Effects of lithium and valproate on hippocampus citrate synthase activity in an animal model of mania. Prog Neuropsychopharmacol Biol Psychiatry 31:887–891
D’Eufemia P, Finocchiaro R, Celli M, Raccio I, Properzi E, Zicari A (2009) Increased nitric oxide release by neutrophils of a patient with tyrosinemia type III. Biomed Pharmacother 63:359–361
Dawson TM, Gonzalez-Zulueta M, Kusel J, Dawson VL (1998) Nitric oxide: diverse actions in the central and peripheral nervous systems. Neuroscientist 4:96–112
de Andrade RB, Gemelli T, Rojas DB, Funchal C, Dutra-Filho CS, Wannmacher CM (2011) Tyrosine inhibits creatine kinase activity in cerebral cortex of young rats. Metab Brain Dis 26:221–227
de Andrade RB, Gemelli T, Rojas DB, Funchal C, Dutra-Filho CS, Wannmacher CM (2012) Tyrosine impairs enzymes of energy metabolism in cerebral cortex of rats. Mol Cell Biochem 364:253–261
Deng H, Hu H, Fang Y (2012) Multiple tyrosine metabolites are GPR35 agonists. Sci Rep 2:373
Fischer JC, Ruitenbeek W, Berden JA, Trijbels JM, Veerkamp JH, Stadhouders AM, Sengers RC, Janssen AJ (1985) Differential investigation of the capacity of succinate oxidation in human skeletal muscle. Clin Chim Acta 153:23–26
García-Cazola A, Wolf IN, Serrano M, Moog U, Perez-Dueñas B, Póo P, Pineda M, Campistol J, Hoffmann GF (2009) Mental retardion and inborn errors of metabolism. J Inherit Metab Dis 32:599–608
Goldsmith LA, Kang E, Bienfang DC, Jimbow K, Gerald P, Baden HP (1973) Tyrosinemia with plantar and palmar keratosis and keratitis. J Pediatr 83:798–805
Halliwell B, Gutteridge JM (2007) Free radicals in biology and medicine. Oxford University Press, New York
Heales SJ, Bolanõs JP, Stewart VC, Brookes PS, Land JM, Clark JB (1999) Nitric oxide, mitochondria and neurological disease. Biochim Biophys Acta 1410:215–228
Held PK (2006) Disorders of tyrosine catabolism. Mol Genet Metab 88:103–106
Jana S, Sinha M, Chanda D, Roy T, Banerjee K, Munshi S, Patro BS, Chakrabarti S (2011) Mitochondrial dysfunction mediated by quinone oxidation products of dopamine: implications in dopamine cytotoxicity and pathogenesis of Parkinson’s disease. Biochim Biophys Acta 1812:663–673
Kelly D, Gordon J, Alpers R, Strauss AW (1989) The tissue-specific expression and developmental regulation of two nuclear genes encoding rat mitochondrial proteins. Medium chain acyl-CoA dehydrogenase and mitochondrial malate dehydrogenase. J Biol Chem 264:18921–18925
Kitto GB (1969) Intra- and extramitochondrial malate dehydrogenases from chicken and tuna heart. Methods Enzymol XIII:106–116
Lemonnier F, Charpentier C, Odievre M, Larregue M, Lemonnier A (1979) Tyrosine aminotransferase isoenzyme deficiency. J Pediatr 94:931–932
Llesuy SF, Milei J, Molina H, Boveris A, Milei S (1985) Comparison of lipid peroxidation and myocardial damage induced by adriamycin and 4’-epiadriamycin in mice. Tumori 71:241–249
Lowry OH, Rosebough NG, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Macsai MS, Schwartz TL, Hinkle D, Hummel MB, Mulhern MG, Rootman D (2001) Tyrosinemia type II: nine cases of ocular signs and symptoms. Am J Ophthalmol 132:522–527
Mitchell GA, Grompe M, Lambert M, Tanguay RM (2001) Hypertyrosinemia. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease. McGraw-Hill, New York, pp 1977–1982
Monsalve M, Borniquel S, Valle I, Lamas S (2007) Mitochondrial dysfunction in human pathologies. Front Biosci 12:1131–1153
Moreira PI, Santos MS, Oliveira CR (2007) Alzheimer’s disease: a lesson from mitochondrial dysfunction. Antioxid Redox Signal 9:1621–1630
Morelli A, Ravera S, Panfoli I (2011) Hypothesis of an energetic function for myelin. Cell Biochem Biophys 61:179–187
Morre MC, Hefti F, Wurtman RJ (1980) Regional tyrosine levels in rat brain after tyrosine administration. J Neural Transm 49:45–50
Nakamura K, Tanaka Y, Mitsubuchi H, Endo F (2007) Aninal models of tyrosinemia. J Nutr 137:1557–1560
Peng ZF, Chen MJ, Yap YW, Manikandan J, Melendez AJ, Choy MS, Moore PK, Cheung NS (2008) Proteasome inhibition: an early or late event in nitric oxide-induced neuronal death? Nitric Oxide 18:136–145
Rabinowitz LG, Williams LR, Anderson CE, Mazur A, Kaplan P (1995) Painful keratoderma and photophobia: hallmarks of tyrosinemia type II. J Pediatr 126:266–269
Rustin P, Chretien D, Bourgeron T, Gérard B, Rötig A, Saudubray JM, Munnich A (1994) Biochemical and molecular investigations in respiratory chain deficiencies. Clin Chim Acta 228:35–51
Schurr A (2002) Energy metabolism, stress hormones and neural recovery from cerebral ischemia/hypoxia. Neurochem Int 41:1–8
Scott CR (2006) The genetic tyrosinemias. Am J Med Genet C Semin Med Genet 142:121–126
Sener RN (2005) Brain magnetic resonance imaging in tyrosinemia. Acta Radiol 46:618–620
Sgaravatti AM, Vargas BA, Zandoná BR, Deckmann KB, Rockenback FJ, Moraes TB, Monserrat JM, Sgarbi MB, Pederzolli CD, Wyse ATS, Wannmacher CMD, Wajner M, Dutra-Filho CS (2008) Tyrosine promotes oxidative stress in cerebral cortex of young rats. Int J Dev Neurosci 26:553–559
Sgaravatti AM, Magnusson AS, de Oliveira AS, Rosa AP, Mescka CP, Zanin FR, Pederzolli CD, Wyse AT, Wannmacher CM, Wajner M, Dutra-Filho CS (2009) Tyrosine administration decreases glutathione and stimulates lipid and protein oxidation in rat cerebral cortex. Metab Brain Dis 24:415–425
Shepherd D, Garland PB (1969) The kinetic properties of citrate synthase from rat liver mitochondria. Biochem J 114:597–610
Shrimpton AE, Braddock BR, Thomson LL, Stein CK, Hoo JJ (2004) Molecular delineation of deletions on 2q37.3 in three cases with an Albright hereditary osteodystrophy-like phenotype. Clin Genet 66:537–544
Stoerner JW, Butler IJ, Morriss FH Jr, Howell RR Jr, Seifert WE Jr, Caprioli RM, Adcock EW, Denson SE (1980) CSF neurotransmitter studies. An infant with ascorbic acid-responsive tyrosinemia. Am J Dis Child 134:492–494
Tyler D (1992) The mitochondrion in health and diseases. VCH, New York
Valikhani M, Akhyani M, Jafari AK, Barzegari M, Toosi S (2005) Oculocutaneous tyrosinaemia or tyrosinaemia type 2: a case report. J Eur Acad Dermatol Venereol 20:591–594
Vincent VA, Tilders FJ, Van Dam AM (1998) Production, regulation and role of nitric oxide in glial cells. Mediators Inflamm 7:239–255
Wajner M, Latini A, Wyse ATS, Dutra-Filho CS (2004) The role of oxidative damage in the neuropathology of organic acidurias: insights from animal studies. J Inherit Metabolic Dis 27:427–448
Acknowledgments
This study was supported by grants from Conselho Nacional de Pesquisa e Desenvolvimento (CNPq), Fundação de Apoio à Pesquisa Científica e Tecnológica do Estado de Santa Catarina (FAPESC) and Universidade do Extremo Sul Catarinense (UNESC).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ferreira, G.K., Scaini, G., Carvalho-Silva, M. et al. Effect of l-Tyrosine In Vitro and In Vivo on Energy Metabolism Parameters in Brain and Liver of Young Rats. Neurotox Res 23, 327–335 (2013). https://doi.org/10.1007/s12640-012-9345-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-012-9345-4