Abstract
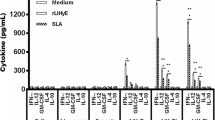
Leishmaniasis is caused by intracellular parasites of Leishmania species, which are transmitted by the bite of the sandfly. Recovery and protection against the infection depends on the induction of a strong Th1 type of immune response. Vaccination of mice with the opioid antagonist naloxone can promote the activation of the Th1 responses. We studied the efficacy of the mixture of naloxone and alum, as an adjuvant, to enhance immune responses and induce protection against Leishmania major infection in BALB/c as a susceptible mouse model. BALB/c mice were immunized with Ag–naloxone–alum, Ag–alum, Ag–naloxone or PBS subcutaneously three times at 2-week intervals. The humoral and cellular specific immune responses were assessed 2 weeks after the last immunization and compared with the control mice. Our results indicated that the administration of alum–naloxone as an adjuvant increased the capability of L. major promastigote antigens to enhance lymphocyte proliferation, the levels of IFN-γ, and the IFN-γ/IL-5 ratio. The results of DTH showed that there were no significant differences in footpad swelling between the groups of immunized mice as compared with the non-vaccinated control group; however, no significant differences were observed in the survival rate among groups. It can be concluded that although immunization with the alum–naloxone mixture in combination with the autoclaved L. major promastigote antigens could enhance cellular immunity and shift the immune response to a Th1 pattern, it could not protect the mice against Leishmania major infection.



Similar content being viewed by others
References
Ameen M (2010) Cutaneous leishmaniasis: advances in disease pathogenesis, diagnostics and therapeutics. Clin Exp Dermatol 35(7):699–705
Badiee A, Jaafari MR, Khamesipour A (2007) Leishmania major: immune response in BALB/c mice immunized with stress-inducible protein 1 encapsulated in liposomes. Exp Parasitol 115(2):127–134
Ebrahimpour S, Tabari MA, Youssefi MR, Aghajanzadeh H, Behzadi MY (2013) Synergistic effect of aged garlic extract and naltrexone on improving immune responses to experimentally induced fibrosarcoma tumor in BALB/c mice. Pharmacogn Res 5(3):189–194
Friedman H, Newton C, Klein TW (2003) Microbial infections, immunomodulation, and drugs of abuse. Clin Microbiol Rev 16(2):209–219
Jamali A, Mahdavi M, Shahabi S, Hassan ZM, Sabahi F, Javan M (2007) Naloxone, an opioid receptor antagonist, enhances induction of protective immunity against HSV-1 infection in BALB/c mice. Microb Pathog 43(5–6):217–223
Jazani NH, Karimzad M, Mazloomi E, Sohrabpour M, Hassan ZM, Ghasemnejad H (2010) Evaluation of the adjuvant activity of naloxone, an opioid receptor antagonist, in combination with heat-killed Listeria monocytogenes vaccine. Microbes Infect 12(5):382–388
Jazani NH, Parsania S, Sohrabpour M, Mazloomi E, Karimzad M, Shahabi S (2011) Naloxone and alum synergistically augment adjuvant activities of each other in a mouse vaccine model of Salmonella typhimurium infection. Immunobiology 216(6):744–751
Karaji AG, Hamzavi Y (2012) The opioid antagonist naloxone inhibits Leishmania major infection in BALB/c mice. Exp Parasitol 130(1):73–77
Manfredi B, Sacerdote P, Bianchi M, Locatelli L, Veljic-Radulovic J, Panerai AE (1993) Evidence for an opioid inhibitory effect on T cell proliferation. J Neuroimmunol 44(1):43–48
McCarthy L, Wetzel M, Sliker JK, Eisenstein TK, Rogers TJ (2001) Opioids, opioid receptors and the immune response. Drug Alcohol Depend 62(2):111–123
Mohammadzadeh Hajipirloo H, Bozorgomid A, Shahabi S, Hazrati Tappeh K, Karamati SA (2014) Evaluation of alum–naltrexone adjuvant activity, on efficacy of anti-leishmania immunization with autoclaved leishmania major (MRHO/IR/75/ER) antigens in BALB/C mice. Iran J Parasitol 9(3):311–318
Molla Hassan AT, Hassan ZM, Moazzeni SM, Mostafaie A, Shahabi S, Ebtekar M (2009) Naloxone can improve the anti-tumor immunity by reducing the CD4+ CD25+ Foxp3+ regulatory T cells in BALB/c mice. Int Immunopharmacol 9(12):1381–1386
Nagill R, Kaur S (2011) Vaccine candidates for leishmaniasis: a review. Int Immunopharmacol 11(10):1464–1488
Panerai AE, Manfredi B, Granucci F, Sacerdote P (1995) The beta-endorphin inhibition of mitogen-induced splenocytes proliferation is mediated by central and peripheral paracrine/autocrine effects of the opioid. J Neuroimmunol 58(1):71–76
Roy S, Ninkovic J, Banerjee S, Charboneau RG, Das S, Dutta R, Kirchner VA (2011) Opioid drug abuse and modulation of immune function: consequences in the susceptibility to opportunistic infections. J Neuroimmune Pharmacol 6(4):442–465
Sacerdote P, Bianchi M, Panerai AE (1996) Involvement of beta-endorphin in the modulation of paw inflammatory edema in the rat. Regul Pept 63(2–3):79–83
Sacerdote P, di San Secondo VE, Sirchia G, Manfredi B, Panerai AE (1998) Endogenous opioids modulate allograft rejection time in mice: possible relation with Th1/Th2 cytokines. Clin Exp Immunol 113(3):465–469
Sacerdote P, Gaspani L, Panerai AE (2000) The opioid antagonist naloxone induces a shift from type 2 to type 1 cytokine pattern in normal and skin-grafted mice. Ann N Y Acad Sci 917:755–763
Shahabi S, Mohammadzadeh Hajipirloo H, Keramati A, Hazrati Tappeh K, Bozorgomid A (2014) Evaluation of the adjuvant activity of propranolol, a beta-adrenergic receptor antagonist, on efficacy of a malaria vaccine model in BALB/c mice. Iran J Allergy Asthma Immunol 13(5):307–316
Singh B, Sundar S (2012) Leishmaniasis: vaccine candidates and perspectives. Vaccine 30(26):3834–3842
Sukumaran B, Madhubala R (2004) Leishmaniasis: current status of vaccine development. Curr Mol Med 4(6):667–679
Acknowledgments
The authors wish to acknowledge the grammatical revision of the manuscript in English facilitated by the Consultation Unit, Office of Publications and Scientometrics, Tehran University of Medical Sciences.
Funding
This study was supported by Urmia University of Medical Sciences, Urmia, Iran (Grant No. 753).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no conflict of interest.
Rights and permissions
About this article
Cite this article
Bozorgomid, A., Hajipirloo, H.M., Tappeh, K.H. et al. Evaluation of the alum–naloxone adjuvant activity against experimental murine leishmaniasis due to L. major . J Parasit Dis 40, 1141–1145 (2016). https://doi.org/10.1007/s12639-015-0731-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12639-015-0731-8