Abstract
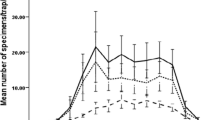
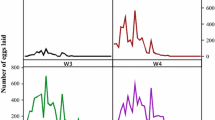
Phlebotomus argentipes Annandale and Brunetti (Diptera: Psychodidae) is the vector for visceral leishmaniasis in India. The aspects of its biology such as feeding and man vector contact are associated with emergence periodicity of the adult. Hence, the present study was made to find out the actual emergence period of P. argentipes. Wild caught P. argentipes were confined in the rearing pots inside laboratory. The newly emerged adults were collected at hourly intervals and released in to separate polythene bags and were held at 4°C till death. Sand flies were segregated sex-wise after the death under a microscope. The emergence of adult was observed throughout the day. However, the male preferred dawn emergence and the female the dusk. Two peaks of emergence were found in a day; first one in the morning (0900h) and the second one in the evening (1800h). The ratio of both sexes was found to be about equal. The emergence of adult was found to be 77% out of total eggs laid, which was completed within 7–10 days from the 1st day of emergence under laboratory conditions (25°C to 31°C and 70% to 75% relative humidity). This study has important bearings to find out the actual time for personal protection against biting of sand flies to prevent the transmission of Kala-azar.
Similar content being viewed by others
References
Botterweg PF (1982) Protandry in the pine looper, Bupaluspiniarius (Lep., Geometridae): an explanatory model. Netherlands J Zool, 32:169–193
Bulmer MG (1983) Models for the evolution of protandry in insects. The Populn Biol, 23:314–322
Carvalho MC, Queiroz PCD, Ruszczy A (1998) Protandry and female size-fecundity variation in the tropical butterfly Brassolis sophorae. Oecologia, 116:98–102
Christina MH, William EP (2002) The relationship between emergence time and male fitness in the pitcher-plant mosquito. Bradshaw Ecol, 83:607–611
Clements AN, Chapman (1992) Development, Nutrition and Reproduction, In: The biology of mosquitoes. Chapman and Hall, London: 509
Dinesh DS, Ranjan A, Palit A, Kishore K, Kar SK (2001) Seasonal and nocturnal landing/biting behaviour of Phlebotomus argentipes (Diptera:Psychodidae). Ann Trop Med Parasitol, 95:197–202
Dinesh DS, Kishore K, Das P, Bhattacharya SK (2005) Abnormality in Phlebotomus argentipes (Diptera:Psychodidae). Curr Sci, 88:703
Dinesh DS, Kishore K, Singh VP, Bhattacharya SK (2005) Morphological variations in Phlebotomus argentipes Annandale and Brunetti (Diptera: Psychodidae). J Commun Dis, 37:35–38
Elgar MA, Pierce NE, Clutton-Brock TH (1988) Mating success and fecundity in an ant-tended lycaenid butterfly. In: Reproductive success. Studies of individual variation in contrasting breeding systems. Univ. of Chicago Press, Chicago, Illinois, USA. 59–75
Fagerstrom T, Wiklund C (1982) Why do males emerge before females? Protandry as a mating strategy in male and female butterflies. Oecologia, 52:164–166
Frottini OP (1973) Entomologia Medica, In: Subfamilia Phlebotominae-Biologia., Edgard Bluncher, Sao Paulo. 4:121–135.
Hastings J (1989) Protandry in western cicada killer wasps (Sphecius grandis, Hymenoptera: Sphecidae): an empirical study of emergence time and mating opportunity. Behavior Ecol and Sociobio, 25:255–260
Hati AK, Ghosh KK, Das SDEN, Sur SA (1981) Longitudinal study on Phlebotomus argentipes/man contact in village in West Bengal. Document WHO/VBC/81.801. Geneva; World Health Organisation
Illango K, Dhanda V, Srinivasan R, Sadanand AV, Lane RP (1994) Phlebotomine sandflies (Diptera: Psychodiae) of Tamil Nadu and Pondicherry, southern India, in relation to leishmaniasis. Ann Trop Med Parasitol, 88:413–431
Illango K (2000) Morphological characteristics of antennal flagellum and its sensilla chaetica with character displacement in sand fly Phlebotomus argentipes Annandale and Brunetti sensu lato (Diptera:Psychodidae). J Biol Sci, 25:163–172
Iwasa Y, Odendaal FJ, Murphy DD, Ehrlich PR, Launer AE (1983) Emergence patterns in male butterflies: a hypothesis and a test. Theor Populn Biol, 23:363–379
Jackson JK (1988) Diel emergence, swarming and longevity of selected adult aquatic insects from a Sonoran Desert stream. Am J Med Nutn, 119:344–352
Jose Francisco LM, Bermudez EGC, Rosa-Freitas MG (2000) Aspects related to productivity for four generations of a Lutzomyia longipalpis laboratory colony. Memo Instit Oswald Cruz Rio De Janeiro, 95:251–257
Justiniano SCB, Chagas AC, Pessoa FAC, Queiroz RG (2004) Comparative biology of two populations of Lutzomyia umbratilis (Diptera:Psychodidae) of Central Amazonia, Brazil, under laboratory conditions. Braz J Biol, 64:227–235
Killick-Kendrick R, Leaney AJ, Ready PD (1977) The establishment, maintenance and productivity of a laboratory colony of Lutzomyia longipalpis (Diptera: Psychodidae). J Med Entomol, 13:429–440
Michener G (1983) Spring emergence schedules and vernal behavior of Richardson’s ground squirrels: Why do males emerge from hibernation before females? Behav Eco Sociobiol, 14:29–38
Parker G, SP Courtney (1983) Seasonal incidence: adaptive variation in the timing of life history stages. J Theor Biol. 105:147–155
Palit A, Chakraborty S, Bhattacharya S, Chowdhury DK, Hati AK (1990) Bait preference of Phlebotomus argentipes (Ann. and Brunn.). Ind J Pub Health 32:41–42
Pinder AM, Trayler KM, Mercer JW, Arena J, Davis JA (1993) Diel periodicities of adult emergence of some chironomids (Diptera: Chironomidae) and a mayfly (Ephemeroptera: Caenidae) at a western Australian wetland. J Austral Entomol, 3:129–135
Pittendrigh CS. 1957. Adaptation, Natural Selection and Behavior. In: A Roe and GG Simson (eds), Behavior and Evolution, New Haven, Yale University Press, 90–416
Srinivasan R, Panicker KN (1992) Biting rhythm and biting activity of Phlebotomid sandflies. Ind J Med Res, (A) 95:301–304
Sawada K, Nomakuchi S, Masumoto T, Suzuki N, Shiotsu Y, Koyanagi H, Sugiura N, Okuda N (1997) Fluctuation of protandry in eclosion of Anthocharis scolymus (Lepidoptera: Pieridae): Can males eclose optimally under evolutionary equilibrium? Environ Entomol, 26:572–579
Swaminath CS, Shortt HE, Anderson LAP (1942) Transmission of Indian Kala-azar to man by bites of Phlebotomus argentipes Ann And Brun India. Ind J Med Res, 30:437–477
Taylor BW, Anderson CR, Peckarsky BL (1998) Effects of size at metamorphosis on stonefly fecundity, longevity, and reproductive success. Oecologia, 114:494–502
Thornhill R, Alcock J (1983) The evolution of insect mating systems. Harvard University Press, Cambridge, Massachusetts, USA
Ward RD (1977) The colonization of Lutzomyia flavicutellata (Diptera:Psychodidae), a vector of Leishmania maxicana amazonensis in Brazil. J Med Entomol, 14:469–476
Wiklund C, Fagerstro T (1977) Why do males emerge before females? Oecologia, 31:153–158
Wiklund C, Lindfors V, Forsberg J (1996) Early male emergence and reproductive phenology of the over wintering butterfly Gonepteryx rhamni in Sweden. Oikos, 75:227–240
Zonneveld C (1992) Polyandry and protandry in butterflies. Bull Math Biol, 54:957–976
Zonneveld C (1996) Being big or emerging early? Polyandry and the trade-off between size and emergence in male butterflies. Am Naturalist, 147:946–965
Zonneveld C, Metz JAJ (1991) Models on butterfly protandry: virgin females are at risk to die. Theor Populn Biol, 14:46–55.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dinesh, D.S., Singh, A., Kumar, V. et al. Emergence periodicity of Phlebotomus argentipes annandale and brunetti (Diptera: psychodidae): A laboratory study. J Parasit Dis 33, 23–27 (2009). https://doi.org/10.1007/s12639-009-0003-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12639-009-0003-6