Abstract
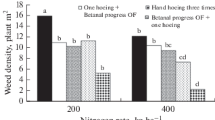
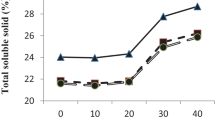
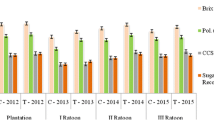
Sugarcane must be processed quickly after being harvested because it is a destructible commodity. Harvested cane may degrade for a variety of reasons, including exposure to microbes, mechanical or manual harvesting, cultivar, maturity, cut-to-crush interval, and storage. Due to the quick loss of sucrose and deterioration after harvest, sugarcane needs to be treated at the right time and way. The higher sugar content of mature internodes offers the perfect conditions for microbial growth, which enters the harvested stalk through wounds or cut ends. The bacteria Leuconostoc spp. is primarily responsible for these post-harvest losses, which negatively affect sugar percent. The trials were carried out to assess the efficacy of Sodium metasilicate (SMS), Benzalkonium chloride (BKC), Nisin (Lactobacteria), and Neem sources on sugarcane for reducing the post-harvest degradation losses. An investigation is underway now to reveal that foliar spray of neem cake @ 5% + dried neem leaves extract @ 5% (in heaping) is the most effective and eco-friendly substance that might be able to significantly enhance sugar recovery. This treatment was comparable with the chemical formulation of SMS @ 2% (3 days before harvest) + BKC @ 2000 ppm (in heaping) which might be a consequence of controlling the proliferation of Leuconostoc spp. bacterium. Likewise, the juice obtained from these treatments has a lower rate of inclination in pH, reducing sugar, total soluble solids, titrable acidity index, invertase activity, higher sucrose, and commercial cane sugars (CCS) recovery, furthermore with relatively smaller losses in cane weight. Hence, these treatments offer a significant potential role in reducing post-harvest deterioration losses in the sugar industry.
Similar content being viewed by others
Data Availability
All relevant data are within the research paper.
References
Upadhyay TK (2021) A study of area, production and productivity of sugarcane in India and Uttar Pradesh. Int J Multi Discip Educ Res 10(1):59–65
Solomon S (2009) Post-harvest deterioration of sugarcane. Sugar Tech 11:109–123
Foster DH, Inkerman PA, Neil KE (1980) Studies on cane deterioration in Australia. Proc.17th Congress ISSCT 3:2204–2220
Singh P, Arya N, Tiwari P, Suman A, Rai RK, Shrivastava AK, Solomon S (2008) J Agric Food Chem 56(16):7176–7183. https://doi.org/10.1021/jf801394j
Solomon S (2002) Post-harvest cane deterioration and its milling consequences. Sugar Tech 2:1–18
Misra V, Solomon S, Shrivastava AK, Shukla SP, Ansari MI (2016) Post-harvest sugarcane deterioration: Leuconostocand its effect. J Funct Environ Bot 6:1–7
Solomon S, Banerji R, Shrivastava AK, Singh P, Singh I, Verma MCP, Prajapati SA (2006) Post-harvest deterioration of sugarcane and chemical methods to minimize sucrose losses. Sugar Tech 8(1):74–78
Singh P, Solomon S, Prajapati CP, Kumar S, Misra V, Chandra A (2014) Dynamics of deterioration of fresh and stale juice in relation to expression of invertases and growth of Leuconostoc sp., Proceedings of green technologies for sustainable growth of sugar and integrated industries in developing countries, Nanning, PR China 120–124
Krishnankumar T, Thamilselvi C, Devadas CT (2013) Effect of delayed extraction and storage on quality of sugarcane. Afr J Agric Res 8:930–935
Solomon S, Singh P (2009) Efficacy of electrolysed water to minimize post-harvest sucrose losses in sugarcane. Sugar Tech 11:228–230
Bruijn J (1966) Deterioration of sugarcane after harvesting part 1, changes in juice composition. Int Sugar J 68:331–334
Misra V, Solomon S, Ansari MI (2016) Impact of drought on post-harvest quality of sugarcane crop. Adv Life Sci 5:9496–9505
Kim B, Robyt JF (1995) Production selection and characterization of mutants of Leuconostoc mesenteroides B742 constitutive of dextransucrase. Enzyme Microbiol Technol 17:689–695
Misra V, Solomon S, Singh P, PrajapatiCP AnsariMI (2016) Effect of water logging on post-harvest sugarcane deterioration. Agrica 5:119–132
McCleskey CS, Faville LW, Barnett RO (1947) Characteristics of Leuconostoc mesenteroidesfrom cane juice. J Bacteriol 54:697–708
Misra V, Mall AK, Pathak AD, Solomon S, Kishor R (2017) Microorganisms affecting post-harvest sucrose losses in sugarcane. Int J Curr Microbiol App Sci 6:2554–2566
Singh P, Solomon S, Prajapati CP, Kumar S, Misra V, Chandra A (2016) Deterioration of fresh and stale cane juice at high ambient temperature in relation to expression of invertases and the growth of Leuconostoc sp. Agrica 4:79–85
Misra V, Solomon S, Hashem A, Abd-Allah EF, Al-Arjani AF, Mall AK, Prajapati CP, Ansari MI (2020) Minimization of post-harvest sucrose losses in drought affected sugarcane using chemical formulation. Saudi J Biol Sci 27:309–317
Huang SX, Hou DZ, Qi PX, Wei YJ, Wang Q, Liang YP, Chen S (2019) Efficacy of neutral electrolyzed water for reducing Leuconostoc mesenteroides in sugarcane mixed juice. Sugar Tech 21:986–994
Zohra RR, Waseem S, Aman A, Siddiqui A, Kazmi SK, Zohra RR (2019) Dextran production by microbial biotransformation of sugarcane waste. FUUAST J Biol 9:87–94
Cuddihy JA, Rauh JS, Porro ME (1998) Improving sugar recovery with sugar process chemicals. http://www.midlandresearchlabsinc.com. Accessed 8 Jul 2004
Sharma KP, Batta SK, Singh R (1994) Studies on minimizing dextran problems in sugarcane under subtropical conditions. Trop Agricult (Trinidad) 71:119–122
Tilbury RH (1975) Occurrence and effects of lactic acid bacteria in the sugar industry. In: Carr JG, Cutting CV, Whiting GC (eds) Whiting lactic acid bacteria in beverages and foods. Academic Press, London, pp 103–128
Holt JG, Krieg NR, Sneath PH, Staley JT, Williams ST (1994) Bergey’s Manual of determinative bacteriology, 9th edn. William & Wilkins, Baltimor, pp 541–529
Nelson N (1944) A photometric adaption of Somogyi method for determination of reducing sugar. J Biol Chem 153:375–380
Hatch MD, Glasziou KT (1963) Sugar-accumulation cycle in sugarcane. II. Relationship of invertase activity to sugar content and growth rate in storage tissue of plants grown in controlled environments. Plant Physiol 38:34
Roe JH, Papadopoulos NM (1954) The determination of fructose6- phosphate and fructose 1, 6 diphosphate. J Biol Chem 210:703
Robert H, Gabriel V, Lefebvre D, Rabier P, Vayssier Y, Faucher CF (2006) Study of the behaviour of Lactobacillus plantarum and Leuconostoc starters during – A complete wheat sourdough bread making process. Lebensmittel -Wissenschaft und-Technologie 39(3):256–265. https://doi.org/10.1016/j.lwt.2005.01.013
Vermeiren L, Devlieghere F, De Graef V, Debevere J (2005) In vitro and in situ growth characteristics and behaviour of spoilage organisms associated with anaerobically stored cooked meat products. J Appl Microbiol 98:33–42
Xiao Z, Liao X, Guo S (2017) Analysis of sugarcane juice quality indexes. J Food Qual. Article ID 1746982, https://doi.org/10.1155/2017/1746982.
Chandra A, Roopendra K, Singh P, Jain R, Prajapati CP, Solomon S (2014) Time-course expression of soluble acid invertase (SAI) gene mirroring post-harvest cane quality deterioration: effective treatments cause reduction of SAI gene expression. Curr Sci 107:184–186
Rakkiyappan P, Shekinah DE, Gopalasundaram P, Mathew MD, Asokan S (2009) Post-harvest deterioration of sugarcane with special reference to quality loss. Sugar Tech 11(2):167–170
Lontom W, Kosittrakun M, Weerathaworn P (2009) Impact of storage temperature and duration on sucrose catabolism in harvested sugarcane stalks. Sugar Tech 11:146–153. https://doi.org/10.1007/s12355-009-0022-8
Bhatia S, Jyoti SK, Uppal KS, Thind SK, Batta (2009) Post-harvest quality deterioration in sugarcane under different environmental conditions. Sugar Tech 11(2):154–160
Saxena P, Srivastava RP, Sharma ML (2010) Impact of cut to crush delay and bio-chemical changes in sugarcane. Aust J Crop Sci 4:692–699
Khan MT, Yasmeen S, Khan IA (2020) Comparative analysis of sugarcane genotypes for post-harvest deterioration under natural conditions. Pak J Bot 52:4
Eggleston G, Huet JM (2012) The measurement of mannitol in beet sugar factories to monitor deterioration and processing problems. Zuckerindustrie Sugar Industry 137(1):33–39
Mao L, Que F, Wang G (2006) Sugar metabolism and involvement of enzymes in sugarcane (Saccharum officinarum L.) stems during storage. Food Chem 98:338–342
Shivalingamurthy SG, Anangi R, Kalaipandian S, Glassop D, King GF, Rae AL (2018) Identification and Functional Characterization of Sugarcane Invertase Inhibitor (ShINH1): A Potential Candidate for Reducing Pre- and Post-harvest Loss of Sucrose in Sugarcane. Front Plant Sci 9:598. https://doi.org/10.3389/fpls.2018.00598
Devi K, Prathima GR, Manimekalai R, Lakshmi K, Selvi A (2019) Gene expression profiling in sugarcane genotypes during drought stress and rehydration. Sugar Tech 21(5):717–733
Mukunda Rao M, Vijaya Kumar M, Sambasiva Rao CH, BalajiNaik R, Sekhar D (2008) Sugarcane quality deterioration between harvest and crushing period under Telangana region of Andhra Pradesh. Coop Sugar 39(12):19–21
Solomon S, Singh P, Shrivastava AK, Singh P, Chandra A, Jain R, Prajapati CP (2011) Physico-chemical method of preserving sucrose in harvested sugarcane at high ambient temperature in a sub-tropical climate. Sugar Tech 13(1):60–67
Uppal SK, Bhatia S, Thind KS (2008) Pre milling cane preparation for high sugar recovery and reduction of post harvest losses in sugarcane. Sugar Tech 10:346–349. https://doi.org/10.1007/s12355-008-0061-6
Misra V, Mall AK, Solomon S, Ansari MI (2022) Post-harvest biology and recent advances of storage technologies in sugarcane. Biotechnol Rep (Amst) 33:e00705. https://doi.org/10.1016/j.btre.2022.e00705
Mohammed HA, Omer AA (2015) Antibacterial activity of Azadirachta indica (Neem) leaf extract against bacterial pathogens in Sudan. Am J Res Com 3(5):246–251
Yilleng TM, Samuel NY, Stephen D, Akande JA, Agendeh ZM, Madaki LA (2020) Biosynthesis of copper and iron nanoparticles using Neem (Azadirachta indica) leaf extract and their anti-bacterial activity. J Appl Sci Environ Manag 24(11):1987–1991
Luthfiah A, Deawati Y, Firdaus ML, Rahayu I, Eddy DR (2021) Silica from natural sources: a review on the extraction and potential application as a supporting photocatalytic material for antibacterial activity. Sci Technol Indonesia 6(3):144–155. https://doi.org/10.26554/sti.2021.6.3.144-155
Smirnov NA, Kudryashov SI, Nastulyavichus AA, Rudenko AA, Saraeva IN, Tolordava ER, Gonchukov SA, Romanova YM, Ionin AA, Zayarny DA (2018) Antibacterial properties of silicon nanoparticles. Laser Phys Lett 15(10):5602. https://doi.org/10.1088/1612-202X/aad853
Tian B, Liu Y (2020) Antibacterial applications and safety issues of silica-based materials: a review. Int J Appl Ceram Technol 18(2):289–301
Anitha R, Vanitha K, Tamilselvi C, Jeyakumar P, Vijayalakshmi D, Yuvaraj M, Nageswari R, Dhanushkodi V, Cyriac J (2023) Potential applications of silicate solubilizing bacteria and potassium silicate on sugarcane crop under drought condition. SILICON. https://doi.org/10.1007/s12633-023-02534-z
Gupta AP, Nigam N (1982) Formation of non-sucrose compounds in sugarcane on storage during post-harvest period. Maharashtra Sugar 7(3):51–64
Acknowledgements
The authors acknowledge Sugarcane Research Station, Cuddalore, Tamil Nadu Agricultural University, India for conducting the research experiment.
Author information
Authors and Affiliations
Contributions
Conceptualization: [R. Anitha, R Brindavathy. P. Jeyakumar] Methodology: [R. Anitha, R. Brindavathy, V. Dhanushkodi, M. Yuvaraj, S. Thiruvarassan] Formal analysis: [R. Anitha, T. Thirumurugan, D. Sassikumar V. Dhanushkodi, S. Thiruvarassan] Investigation: [R. Anitha, M. Jayachandran T.Thirumurugan, P. Jeyakumar] Writing–original draft: [R. Anitha, N. Sritharan T.Thirumurugan, P. Jeyakumar, N. Jagathjothi, R. Sathya Priya] Writing review and editing: [R. Anitha, N.Sritharan P. Jeyakumar M. Yuvaraj, C. Jaiby, T. Thirumurugan]; Supervision: [R. Anitha, M. Jayachandran, D. Sassikumar N. Sritharan, K.B. Sujatha].
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Consent was obtained from every researcher who participated in the experiment.
Consent for Publication
The authors have given permission for this research paper to be published in the journal.
Competing Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Anitha, R., Brindavathy, R., Sritharan, N. et al. Comparative Efficacy of Sodium Metasilicate and Organic Source Combination on Sugarcane (Saccharum officinarum L.) for Reducing the Post-harvest Deterioration Losses. Silicon 16, 425–434 (2024). https://doi.org/10.1007/s12633-023-02679-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12633-023-02679-x