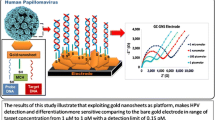
Abstract
We have developed porous silicon (PSi) biosensors for detecting high-risk human papilloma virus 16 and 18, (HPV16 and HPV18), frequently associated with the generation of pre-cancerous and cancerous lesions. For this purpose, we used PSi, a biocompatible material characterized by its remarkable optical properties and porous structure that provides an easily modifiable surface. Here, ssDNA oligonucleotides of HPV 16 and 18 were attached inside PSi pores using standard amino-silane and glutaraldehyde chemistry (PSiMc/HPV-ssDNA). The PSiMc/HPV-ssDNA device was characterized during all the modification steps by infrared spectroscopy (FTIR) and scanning electron microscopy (SEM) to have reliable information about the biosensor’s chemistry and stability. Molecular binding on the PSi surface was optically detected based on a shift of the reflectance spectra and corroborated by fluorescence microscopy. The magnitude of the resonance shift was directly related to the concentration of biomolecules attached to the pore walls. It was found that the PSi biosensor can selectively discriminate between complementary and non-complementary DNA. These studies suggest that the proposed screening strategy based on PSiMc/HPV-ssDNA-ccDNA using reflectance spectra shift may be suitable for the design of novel and practical portable devices for HPV detection. To perform this work, we have selected a PSi microcavity, mainly due to its optical features (resonance modes) in the reflectance spectra that allow the detection of material infiltrated into the porous structure.
Similar content being viewed by others
Data Availability
Not applicable for that section.
References
Cancer Today IARC. https://gco.iarc.fr/today/home. Accessed 6 May 2022
Chandra S, Barola N, Bahadur D (2011) Impedimetric biosensor for early detection of cervical cancer. Chem Commun 47:11258–11260. https://doi.org/10.1039/c1cc14547a
Li H, Wu X, Cheng X (2016) Advances in diagnosis and treatment of metastatic cervical cancer. J Gynecol Oncol 27:1–20. https://doi.org/10.3802/jgo.2016.27.e43
WHO/PAHO. https://www.paho.org/en/topics/cervical-cancer. Accessed 6 May 2022
WHO Human Papilloma Virus. https://www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer. Accessed 6 May 2022
Nilyanimit P, Wanlapakorn N, Niruthisard S, Takahashi M, Vongpunsawad S, Poovorawan Y (2014) Comparison of detection sensitivity for human papillomavirus between self-collected vaginal swabs and physician-collected cervical swabs by electrochemical DNA chip. Asian Pac J Cancer Prev 15:10809–10812. https://doi.org/10.7314/APJCP.2014.15.24.10809
Michelow P, Sherrin A, Rossouw L, Mohaleamolla S, Evans D, Swarts A, Rakhombe N, Smith JS, Firnhaber C (2016) Performance of the Cellslide® automated liquid-based cytology system amongst HIV-positive women. Afr J Lab Med 5:1–6. https://doi.org/10.4102/ajlm.v5i1.278
Saleh HS (2014) Can visual inspection with acetic acid be used as an alternative to Pap smear in screening cervical cancer? Middle East Fertil Soc J 19:187–191. https://doi.org/10.1016/j.mefs.2013.10.003
Jeronimo J, Castle PE, Herrero R, Burk RD, Schiffman M (2003) HPV testing and visual inspection for cervical cancer screening in resource-poor regions. Int J Gynecol Obstet 83:311–313. https://doi.org/10.1016/S0020-7292(03)00299-6
Brink AATP, Snijders PJF, Meijer CJLM (2007) HPV detection methods. Dis Markers 23:273–281. https://doi.org/10.1155/2007/147429
Szuhai K, Sandhaus E, Kolkman-Uljee SM, Lemaître M, Truffert JC, Dirks RW, Tanke HJ, Fleuren GJ, Schuuring E, Raap AK (2001) A novel strategy for human papillomavirus detection and genotyping with sybrgreen and molecular beacon polymerase chain reaction. Am J Pathol 159:1651–1660. https://doi.org/10.1016/S0002-9440(10)63012-X
Matah M, Sareen S (2012) Detection of HPV by PCR-A novel step in the prevention of cancer cervix. J Obstet Gynecol India 62:188–191. https://doi.org/10.1007/s13224-012-0167-3
Poljak M, Marin IJ, Seme K, Vince A (2002) Hybrid Capture II HPV Test detects at least 15 human papillomavirus genotypes not included in its current high-risk probe cocktail. J Clin Virol 25(3):S89–S97. https://doi.org/10.1016/S1386-6532(02)00187-7
Zhao X, Wu Q, Wang X, Fu Y, Zhang X, Tian X, Cheng B, Lu B, Yu X, Lan S, Lu W, Ma D, Cheng X, Xie X (2018) The performance of human papillomavirus DNA detection with type 16/18 genotyping by hybrid capture in primary test of cervical cancer screening: a cross-sectional study in 10,669 Chinese women. Clin Microbiol Infect 24:1322–1327. https://doi.org/10.1016/j.cmi.2018.02.027
Jampasa S, Siangproh W, Laocharoensuk R, Yanatatsaneejit P, Vilaivan T, Chailapakul O (2018) A new DNA sensor design for the simultaneous detection of HPV type 16 and 18 DNA. Sens Actuators B Chem 265:514–521. https://doi.org/10.1016/j.snb.2018.03.045
Herold BC, Keller MJ, Shi Q, Hoover DR, Carpenter CA, Huber A, Parikh UM, Agnew KJ, Minkoff H, Colie C, Nowicki MJ, D’Souza G, Watts DH, Anastos K (2013) Plasma and mucosal HIV viral loads are associated with genital tract inflammation in HIV-infected women. J Acquir Immune Defic Syndr 63:485–493. https://doi.org/10.1097/QAI.0b013e3182961cfc
Sandra Olimpia G-E, Víctor Omar F-N, Cuauhtémoc O-O, Julio Alejandro O-V, Darío G-H, Yolanda T-F (2013) Training of health personnel to improve knowledge and skills in taking Pap: Effect of an educational intervention to prevent cervical cancer. Health (Irvine Calif) 05:60–64. https://doi.org/10.4236/health.2013.57a4009
Andrade D, Vanwagenen A, Gregonis E (1985) Remote fiber-optic biosensors based on evanescent-excited fluoro-inmunuassay: concept and progress. IEEE Trans Electron Devices 32:1175–1179. https://doi.org/10.1109/TED.1985.22096
Burd EM (2016) Human papillomavirus laboratory testing: the changing paradigm, 29291–319. https://doi.org/10.1128/CMR.00013-15
Daniel RW, Ahdieh L, Hayden D, Cu-Uvin S, Shah KV (2000) Intra-laboratory reproducibility of human papillomavirus identification in cervical specimens by a polymerase chain reaction-based assay. J Clin Virol 19:187–193. https://doi.org/10.1016/S1386-6532(00)00142-6
Castle PE, Wacholder S, Sherman ME, Lorincz AT, Glass AG, Scott DR, Rush BB, Demuth F, Schiffman M (2002) Absolute risk of a subsequent abnormal Pap among oncogenic human papillomavirus DNA-positive, cytologically negative women. Cancer 95:2145–2151. https://doi.org/10.1002/cncr.10927
Lowe B, Kobayashi L, Lorincz A, Mallonee R, O’Neil D, Thai H, Nazarenko I (2010) HPV genotype detection using hybrid capture sample preparation combined with whole genome amplification and multiplex detection with luminex XMAP. J Mol Diagnostics 12:847–853. https://doi.org/10.2353/jmoldx.2010.100045
Patra PD (2010) Cervical cancer screening in developing countries. Indian J Cancer 47:344–435. https://doi.org/10.1016/S1068-607X(00)00032-9
De Stefano L, Arcari P, Lamberti A, Sanges C, Rotiroti L, Rea I, Rendina I (2007) DNA optical detection based on porous silicon technology: From biosensors to biochips. Sensors 7:214–221. https://doi.org/10.3390/s7020214
Terracciano M, Rea I, Borbone N, Moretta R, Oliviero G, Piccialli G, De Stefano L (2019) Porous silicon-based aptasensors: The next generation of label-free devices for health monitoring. Molecules 24. https://doi.org/10.3390/molecules24122216
Rahimi F, Fardindoost S, Ansari-Pour N, Sepehri F, Makiyan F, Shafiekhani A, Rezayan AH (2019) Optimization of porous silicon conditions for DNA-based biosensing via reflectometric interference spectroscopy. Cell J 20:584–591. https://doi.org/10.22074/cellj.2019.5598
Palestino G, Agarwal V, Aulombard R, Pérez E, Gergely C (2008) Biosensing and protein fluorescence enhancement by functionalized porous silicon devices. Langmuir 24:13765–13771. https://doi.org/10.1021/la8015707
Palestino G, Martin M, Agarwal V, Legros R, Cloitre T, László Zimányi C, Gergely (2009) Detection and light enhancement of glucose oxidase adsorbed on porous silicon microcavities. Phys Status Solidi Curr Top Solid State Phys 6:1624–1628. https://doi.org/10.1002/pssc.200881006
De La Cruz-Guzman M, Aguilar A, Bañuelos-Frias A, Chazaro-Ruiz LF, Palestino G (2014) Hybrid porous silicon-Rhodamine B derivative nanostructures as chemical sensor for hg(ii) detection. ECS Trans 64:31–34. https://doi.org/10.1149/06401.0031ecst
Palestino G, Legros R, Agarwal V, Pérez E, Gergely C (2008) Functionalization of nanostructured porous silicon microcavities for glucose oxidase detection. Sens Actuators B Chem 135:27–34. https://doi.org/10.1016/j.snb.2008.07.013
Tölli MA, Ferreira MPA, Kinnunen SM, Rysä J, Mäkilä EM, Szabó Z, Serpi RE, Ohukainen PJ, Välimäki MJ, Correia AMR, Salonen JJ, Hirvonen JT, Ruskoaho HJ, Santos HA (2014) In vivo biocompatibility of porous silicon biomaterials for drug delivery to the heart. Biomaterials 35:8394–8405. https://doi.org/10.1016/j.biomaterials.2014.05.078
Zhao Y, Lawrie JL, Beavers KR, Laibinis PE, Weiss SM (2014) Effect of DNA-induced corrosion on passivated porous silicon biosensors. ACS Appl Mater Interfaces 6:13510–13519. https://doi.org/10.1021/am502582s
Steinem C, Janshoff A, Lin VSY, Völcker NH, Reza M, Ghadiri (2004) DNA hybridization-enhanced porous silicon corrosion: Mechanistic investigations and prospect for optical interferometric biosensing. Tetrahedron 60:11259–11267. https://doi.org/10.1016/j.tet.2004.06.130
Palestino AG, De La Mora MB, Del JA, Río C, Gergely E, Ṕrez (2007) Fluorescence tuning of confined molecules in porous silicon mirrors. Appl Phys Lett 91:2005–2008. https://doi.org/10.1063/1.2786596
Franco EL, Duarte-Franco E, Ferenczy A (2001) Cervical cancer: Epidemiology, prevention and the role of human papillomavirus infection. CMAJ 164:1017–1025. https://doi.org/10.1016/j.vaccine.2008.05.064.Epidemiology
Rocha-García D, Betancourt-Mendiola MdeL, Wong-Arce A, Rosales-Mendoza S, Reyes-Hernández J, González-Ortega O, Palestino G (2018) Gelatin-based porous silicon hydrogel composites for the controlled release of tramadol. Eur Polym J 108:485–497. https://doi.org/10.1016/j.eurpolymj.2018.09.033
Chen SH, Lin KI, Tang CY, Peng SL, Chuang YC, Lin YR, Wang JP, Lin CS (2009) Optical detection of human papillomavirus type 16 and type 18 by sequence sandwich hybridization with oligonucleotide-functionalized au nanoparticles. IEEE Trans Nanobioscience 8:120–131. https://doi.org/10.1109/TNB.2008.2011733
Janshoff A, Dancil KPS, Steinem C, Greiner DP, Lin VSY, Gurtner C, Motesharei K, Sailor MJ, Reza M, Ghadiri (1998) Macroporous p-type silicon Fabry-Perot layers. Fabrication, characterization, and applications in biosensing. J Am Chem Soc 120:12108–12116. https://doi.org/10.1021/ja9826237
Dzuba IG, Díaz EY, Allen B, Leonard YF, Ponce ECL, Shah KV, Bishai D, Lorincz A, Ferris D, Turnbull B, Avila MH, Salmerón J (2002) The acceptibility of self-collected samples for HPV testing vs. The pap test as alternatives in cervical cancer screening, J. Women’s Heal. Gender-Based Med 11:265–275. https://doi.org/10.1089/152460902753668466
Terán-Figueroa Y, Muñiz-Carreón P, Moya MF, Galán-Cuevas S, Noyola-Range N, Gutiérrez-Enríquez SO, Ortiz-Valdez JA (2015) Cruz-Valdez, Repercusión del cancer cervicouterino en pacientes con limitaciones de acceso a los servicios de salud. Ginecol Obstet Mex 83:162–172
Madzima TR, Vahabi M, Lofters A (2017) Clinical review emerging role of HPV self-sampling in cervical cancer screening for hard-to-reach women focused literature review. Can Fam Physician 63:597–601
QIAGEN (2015) digene ® HC2 High-Risk HPV DNA Test instructions for use. pp 1–128. https://www.qiagen.com/us/products/diagnostics-and-clinicalresearch/sexual-reproductive-health/cervical-cancer-screening/digene-hc2-highrisk-hpv-dna-test/
Howard FB, Chen C, Cohen JS, Miles HT (1984) Poly(d2NH2A-dT): Effect of 2-amino substituent on the B to Z transition, Biochem. Biophys Res Commun 118:848–853. https://doi.org/10.1016/0006-291X(84)91472-4
Vamvakaki V, Hatzimarinaki M, Chaniotakis N (2008) Biomimetically synthesized silica−carbon nanofiber architectures for development of highly stable electrochemical biosensor systems. Anal Chem 80:5970–5975. https://doi.org/10.1021/ac800614j
Cervical Cancer (2019) PAHO WHO. https://www.paho.org/en/topics/cervical-cancer. Accessed 6 May 2022
Hooper JE, Hebert JF, Schilling A, Gross ND, Joshua S, Lagowski JP, Kulesz-martin M, Ph D, Christopher L, Ph D, Morgan TK, Ph D (2015) Hybrid capture 2 is as effective as PCR testing for high risk human papillomavirus in head and neck cancers. HHS Public Access 23:266–272. https://doi.org/10.1097/PDM.0000000000000036.Hybrid
García-Carrancá A (2003) Vaccines against human papillomavirus and perspectives for the prevention and control of cervical cancer. Salud Publica Mex 45:437–442. https://doi.org/10.1590/s0036-36342003000900018
Harper DM, Nieminen P, Paavonen J, Lehtinen M (2010) Cervical cancer incidence can increase despite HPV vaccination. Lancet Infect Dis 10:594–595. https://doi.org/10.1016/S1473-3099(10)70182-1
Jimenez Jimenez AM, Rodrigo MAM, Milosavljevic V, Krizkova S, Kopel P, Heger Z, Adam V (2017) Gold nanoparticles-modified nanomaghemite and quantum dots-based hybridization assay for detection of HPV. Sens Actuators B Chem 240:503–510. https://doi.org/10.1016/j.snb.2016.08.091
Jimenez Jimenez AM, Moulick A, Richtera L, Krejcova L, Kalina L, Datta R, Svobodova M, Hynek D, Masarik M, Heger Z, Adam V (2018) Dual-color quantum dots-based simultaneous detection of HPV-HIV co-infection. Sens Actuators B Chem 258:295–303. https://doi.org/10.1016/j.snb.2017.11.074
Teengam P, Siangproh W, Tuantranont A, Henry CS, Vilaivan T, Chailapakul O (2017) Electrochemical paper-based peptide nucleic acid biosensor for detecting human papillomavirus. Anal Chim Acta 952:32–40. https://doi.org/10.1016/j.aca.2016.11.071
Acknowledgements
The authors are grateful to CONACYT México for research grant. The authors thanks to Ph.D Candida Anahy Cisneros-Covarrubias and Ch.E. Ana Lourdes Rodríguez-Villanueva for the analytical and technical support. We also thanks to Professor Amaury de Jesus Pozos-Guillen and Professor Jaime Ruiz-García for the facilities given to use the confocal and fluorescent microscopes.
Funding
This research was supported by CONACYT-MEXICO, CB 2017/2018 A1-S-31287 grant given to GP.
Author information
Authors and Affiliations
Contributions
All authors reviewed the manuscript. Material preparation, data collection and analysis were performed by S. R. M., D. S. M. G. and A. B. F. Figures and edition were performed by A. B. F. and C.F.A.G.D. The first draft of the manuscript was written by all authors in different sections. All authors commented and contributed on previous versions of the manuscript. Revision and writing were performed by Y.T.F. and G.P. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable for that section.
Consent to Participate
Not applicable for that section.
Consent for Publication
Not applicable for that section.
Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rodríguez-Montelongo, S.A., Moreno-Gutiérrez, D.S., Terán-Figueroa, Y. et al. Porous Silicon-Based DNA Biosensor for Human Papillomavirus Detection: Towards the Design of Fast and Portable Test. Silicon 15, 2371–2383 (2023). https://doi.org/10.1007/s12633-022-02179-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12633-022-02179-4