Abstract
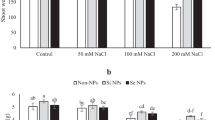
Salinity is one of the most crucial abiotic stresses, which is the consequence of an increase in the concentration of NaCl ions, influencing the plant’s growth, development, and yield. The present study was aimed to evaluate the effects of methyl jasmonate (MeJA) (0.5 mM) and silicon nanoparticles (2 mM) on physiological characteristics and spatial expression patterns of some salinity-related genes (DREB, cAPX, Mn-SOD, and GST) in strawberry cv. Paros under saline (50 mM NaCl) and non-saline conditions, at 12, 24, and 36 h after treatments’ initiation. Based on the results, salinity increased the content of H2O2, proline and the activity of SOD in a time-dependent manner, while it decreased the percentage of MSI and the content of total soluble protein (TSP). In contrast, the implementation of Si nanoparticles and MeJA not only decreased H2O2 and maintained MSI percentage, but they also recovered TSP content and increased the transcription level of DREB, cAPX, MnSOD, and GST genes. We concluded that strawberry plants had a better response to salinity by enhancing both enzymatic and non-enzymatic antioxidant physiological protection mechanisms and also by increasing the transcription of salinity-related genes upon MeJA and Si application.
Similar content being viewed by others
Data Availability
All data and software application support their published claims and comply with field standards.
References
Grattan SR, Grieve CM (1998) Salinity–mineral nutrient relations in horticultural crops. Sci Hort 78(1):127–157
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Isayenkov SV, Maathuis FJ (2019) Plant salinity stress: many unanswered questions remain. Front Plant Sci 10:80
Yaghubi K et al (2016) Potassium silicate alleviates deleterious effects of salinity on two strawberry cultivars grown under soilless pot culture. Sci Hort 213:87–95
Hasegawa PM et al (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Biol 51(1):463–499
Gupta B, Huang B (2014) Mechanism of salinity tolerance in plants: physiological, biochemical, and molecular characterization. Int J Genomics 701596:1–18
Rao AQ et al (2016) Genomics of salinity tolerance in plants. In: Plant Genomics, I. Y. Abdurakhmono, Editor. IntechOpen, London
Mazzoni L et al (2020) Sensorial and nutritional quality of inter and intra—Specific strawberry genotypes selected in resilient conditions. Sci Hort 261:108945
Sandhu D et al (2019) Variable salinity responses and comparative gene expression in woodland strawberry genotypes. Sci Hort 254:61–69
Orsini F et al (2012) Low stomatal density and reduced transpiration facilitate strawberry adaptation to salinity. Environ Exp Bot 81:1–10
Yaghubi K et al (2019) Potassium silicate improves salinity resistant and affects fruit quality in two strawberry cultivars grown under salt stress. Commun Soil Sci Plant Anal 50(12):1439–1451
Garriga M et al (2015) Effect of salt stress on genotypes of commercial (Fragaria x ananassa) and Chilean strawberry (F. chiloensis). Sci Hortic 195(195):37–47
Christou A et al (2013) Hydrogen sulfide induces systemic tolerance to salinity and non-ionic osmotic stress in strawberry plants through modification of reactive species biosynthesis and transcriptional regulation of multiple defence pathways. J Exp Bot 64(7):1953–1966
Basu S, Roychoudhury A (2014) Expression profiling of abiotic stress-inducible genes in response to multiple stresses in rice (Oryza sativa L.) varieties with contrasting level of stress tolerance. BioMed Res Int 2014:706890
Shinozaki K, Yamaguchi-Shinozaki K (2007) Gene networks involved in drought stress response and tolerance. J Exp Bot 58(2):221–227
Guo Q et al (2019) Heterologous expression of the DREB transcription factor AhDREB in Populus tomentosa Carrière confers tolerance to salt without growth reduction under greenhouse conditions. Forests 10(3):214
Nakashima K et al (2000) Organization and expression of two Arabidopsis DREB2 genes encoding DRE-binding proteins involved in dehydration- and high-salinity-responsive gene expression. Plant Mol Biol 42:657–665
Franzoni G et al (2019) Transcriptional regulation in rocket leaves as affected by salinity. Plants 9(1):20
Chan C, Lam H-M (2014) A putative lambda class glutathione s-transferase enhances plant survival under salinity stress. Plant Cell Physiol 55(3):570–579
Lin K, Pu S (2010) Tissue-and genotype-specific ascorbate peroxidase expression in sweet potato in response to salt stress. Biol Plant 54(4):664–670
Jha B, Sharma A, Mishra AJ (2011) Expression of SbGSTU (tau class glutathione S-transferase) gene isolated from Salicornia brachiata in tobacco for salt tolerance. Mol Biol Rep 38(7):4823–4832
Ji W et al (2010) Over-expression of a glutathione S-transferase gene, GsGST, from wild soybean (Glycine soja) enhances drought and salt tolerance in transgenic tobacco. Biotechnol Lett 32(8):1173–1179
Saibi W, Brini F (2018) Superoxide dismutase: structure, synthesis applications. In: Superoxide dismutase (SOD) and abiotic stress tolerance in plants: An overview, S. Magliozzi, Editor, p 101–142
Feng K et al (2016) The SOD gene family in tomato: identification, phylogenetic relationships, and expression patterns. Front Plant Sci 7:1279
Bayanati M et al (2019) Expression patterns analysis of SOD genes in responses to ethylene-induced oxidative stress in rose (Rosa hybrida) during flower development. South Afr J Bot 127:265–270
Hernández JA et al (2000) Tolerance of pea (Pisum sativum L.) to long-term salt stress is associated with induction of antioxidant defences. Plant Cell Environ 23(8):853–862
Nazar R, Umar S, Khan NA (2015) Exogenous salicylic acid improves photosynthesis and growth through increase in ascorbate-glutathione metabolism and S assimilation in mustard under salt stress. Plant Signal Behav 10(3):e1003751–e1003751
Auobi Amirabad S, Behtash F, Vafaee Y (2020) Selenium mitigates cadmium toxicity by preventing oxidative stress and enhancing photosynthesis and micronutrient availability on radish (Raphanus sativus L.) cv. Cherry Belle. Environ Sci Pollut Res 27(11):12476–12490
Hernandez JA (2019) Salinity tolerance in plants: trends and perspectives. Int J Mol Sci 20(10):2408
Apel K, Hirt H (2004) Reactive Oxygen Species: Metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55(1):373–399
Lu Z, Liu D, Liu S (2007) Two rice cytosolic ascorbate peroxidases differentially improve salt tolerance in transgenic Arabidopsis. Plant Cell Rep 26(10):1909–1917
Davletova S et al (2005) Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell 17(1):268
Broadley M, Marschner P et al (2002) Benifitical elements. Marschner’s Mineral Nutrition of Higher Plants. Academic Press, San Diego, pp 249–269
Coskun D et al (2016) The role of silicon in higher plants under salinity and drought stress. Front Plant Sci 7:1072
Al Murad M, Khan AL, Muneer S (2020) Silicon in horticultural crops: cross-talk, signaling, and tolerance mechanism under salinity stress. Plants 9(4):460
Ahmad P et al (2016) Jasmonates: multifunctional roles in stress tolerance. Front Plant Sci 7:813
Ali MS, Baek K-H (2020) Jasmonic acid signaling pathway in response to abiotic stresses in plants. Int J Mol Sci 21(2):621
Faghih S, Ghobadi C, Zarei A (2017) Response of strawberry plant cv. ‘Camarosa’ to salicylic acid and methyl jasmonate application under salt stress condition. J Plant Growth Regul 36(3):651–659
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Circ Calif Agr Expt Stat 347(347):1–32
Sairam RK (1994) Effect of moisture stress on physiological activities of two contrasting wheat genotypes. Indian J Exp Biol 32(32):594–597
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(72):248–254
Beauchamp C, Fridovich I (1971) Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal Biochem 44(44):276–287
Mazzara M, James DJ (2000) The influence of photoperiodic growth condition on isolation of RNA from strawberry (Fragaria × ananassa Duch.) tissue. Mol Biotechnol 15(3):237–241
Geng Y et al (2013) A Spatio-temporal understanding of growth regulation during the salt stress response in Arabidopsis. Plant Cell 25(6):2132–2154
Liang Y et al (2003) Exogenous silicon (Si) increases antioxidant enzyme activity and reduces lipid peroxidation in roots of salt-stressed barley (Hordeum vulgare L.). J Plant Physiol 160(10):1157–1164
Abdel-Haliem MEF et al (2017) Effect of silica ions and nano silica on rice plants under salinity stress. Ecol Eng 99:282–289
Ma JF, Yamaji N (2006) Silicon uptake and accumulation in higher plants. Trends Plant Sci 11(8):392–397
Haghighi M, Pessarakli M (2013) Influence of silicon and nano-silicon on salinity tolerance of cherry tomatoes (Solanum lycopersicum L.) at early growth stage. Sci Hort 161:111–117
Farhangi-Abriz S, Torabian S (2018) Nano-silicon alters antioxidant activities of soybean seedlings under salt toxicity. Protoplasma 255(3):953–962
Kazan K (2015) Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends Plant Sci 20(4):219–229
Wang J et al (2020) Functions of jasmonic acid in plant regulation and response to abiotic stress. Int J Mol Sci 21(4):1446
Arbona V, Gomez-Cadenas A (2008) Hormonal modulation of Citrus responses to flooding. J Plant Growth Regul 27:241–250
Qiu Z et al (2014) Exogenous jasmonic acid can enhance tolerance of wheat seedlings to salt stress. Ecotoxicol Environ Saf 104:202–208
Shavrukov Y et al (2016) Expression level of the DREB2-type gene, identified with amplifluor SNP markers, correlates with performance, and tolerance to dehydration in bread wheat cultivars from Northern Kazakhstan. Front Plant Sci 7:1736
Dubouzet J et al (2003) Isolation and functional characterization of the DREB family of genes in rice. In: Advances in Rice Genetics: (In 2 Parts). World Scientific, p 406–409
Pandey GK et al (2015) Analyzing the expression profile of AREB/ABF and DREB/CBF genes under drought and salinity stresses in grape (Vitis vinifera L.). Plos One 10(7): e0134288
Muneer S, Jeong BR (2015) Proteomic analysis of salt-stress responsive proteins in roots of tomato (Lycopersicon esculentum L.) plants towards silicon efficiency. Plant Growth Regul 77(2):133–146
Khattab HI et al (2014) Effect of selenium and silicon on transcription factors NAC5 and DREB2A involved in drought-responsive gene expression in rice. Biol Plant 58(2):265–273
Attia H et al (2008) Long-term effects of mild salt stress on growth, ion accumulation and superoxide dismutase expression of Arabidopsis rosette leaves. Physiol Plant 132(3):293–305
Sheteiwy MS et al (2020) Seed priming and foliar application with Jasmonic acid enhance salinity stress tolerance of soybean (Glycine max L.) seedlings. J Sci Food Agric 101(5):2027–2041
Ahmad B et al (2019) Role of methyl Jasmonates in salt stress tolerance in plants. In: Plant signaling molecules: Role and regulation under stressful environments, M Iqbal Khan, P S Reddy, A Ferrante, N Khan, Editors, Woodhead Publishing
Zhang Y et al (2015) Clones of FeSOD, MDHAR, DHAR genes from white clover and gene expression analysis of ROS-scavenging enzymes during abiotic stress and hormone treatments. Molecules 20(11):20939–20954
Hu L et al (2012) Responses of antioxidant gene, protein and enzymes to salinity stress in two genotypes of perennial ryegrass (Lolium perenne) differing in salt tolerance. J Plant Physiol 169(2):146–156
Rasool S et al (2013) Changes in growth, lipid peroxidation and some key antioxidant enzymes in chickpea genotypes under salt stress. Acta Physiol Plant 35(4):1039–1050
Riemann M et al (2015) Exploring Jasmonates in the hormonal network of drought and salinity responses. Front Plant Sci 6:1077
Ma D et al (2015) Silicon Application Alleviates Drought Stress in Wheat Through Transcriptional Regulation of Multiple Antioxidant Defense Pathways. J Plant Growth Regul 35(1):1–10
Zhu Y et al (2019) Transcriptomic dynamics provide an insight into the mechanism for silicon-mediated alleviation of salt stress in cucumber plants. Ecotoxicol Environ Saf 174:245–254
Walia H et al (2006) Expression analysis of barley (Hordeum vulgare L.) during salinity stress. Funct Integr Genomics 6(2):143–156
Dinler BS, Antoniou C, Fotopoulos V (2014) Interplay between GST and nitric oxide in the early response of soybean (Glycine max L.) plants to salinity stress. J Plant Physiol 171(18):1740–1747
Yang G et al (2014) Overexpression of a GST gene (ThGSTZ1) from Tamarix hispida improves drought and salinity tolerance by enhancing the ability to scavenge reactive oxygen species. Plant Cell Tissue Organ Cult (PCTOC) 117(1):99–112
Csiszár J et al (2014) Glutathione transferase supergene family in tomato: salt stress-regulated expression of representative genes from distinct GST classes in plants primed with salicylic acid. Plant Physiol Biochem 78:15–26
Hu T et al (2011) Isolation and characterization of a rice glutathione S-transferase gene promoter regulated by herbicides and hormones. Plant Cell Rep 30(4):539–549
Author information
Authors and Affiliations
Contributions
YV and AKM participated in its design and coordination, and guaranteed of integrity of the entire study. PM carried out the experiment. YV performed statistical analysis. YV and NAT were involved in drafting the manuscript, evaluated the statistical analysis and critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent to Participate
All authors consent to participate to this publication.
Consent for Publication
Not applicable.
Ethics Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Moradi, P., Vafaee, Y., Mozafari, A.A. et al. Silicon Nanoparticles and Methyl Jasmonate Improve Physiological Response and Increase Expression of Stress-related Genes in Strawberry cv. Paros Under Salinity Stress. Silicon 14, 10559–10569 (2022). https://doi.org/10.1007/s12633-022-01791-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12633-022-01791-8