Abstract
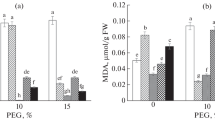
Drought stress limits the growth and yield of essential oils of valuable medicinal plants such as feverfew (Tanacetum parthenium). So, foliar application efficiency of nano-silicon complexes (glycine-nano-silicon, histidine-nano-silicon, glutamine-nano-silicon) was assessed on the growth and yield of feverfew, in a 120-day period of drought stress that was imposed by different irrigation gaps (4, 8 and 12 days). Nano-silicon compounds were sprayed on plants at three concentrations (zero-control, 1.5 and 3.0 mM) at 10-day intervals. The results showed that with increasing intensity of drought stress, leaf water saturation, accumulation of hydrogen peroxide and malondialdehyde increased and membrane damage in the leaf developed. With the loss of leaf water content and the development of oxidative damage, biomass accumulation, leaf area, the number of flowering branches in the plant decreased. Under severe drought stress, antioxidant capacity, phenol accumulation and essential oil yield decreased. The application of nano-silicon complexes limited tissue dehydration (35%) and the development of oxidative damage (30%) under drought stress conditions and restored the growth (25%) and yield of plant essential oils (30%). There was no significant difference between 1.5 and 3.0 mM nano-silicon compounds in most of the evaluated indices and glycine-nano-silicon combination was most effective in increasing plant drought tolerance.
Similar content being viewed by others
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Pareek A, Suthar M, Rathore GS, Bansal V (2011) Feverfew (Tanacetum parthenium L.): A systematic review. Pharmacogn Rev 5 (9):103–110
Ahmadi Z, Ghorbanpour M, Hadian J, Salehi Arjomand H (2018) Effect of chromiumnanocarbon spraying solution and salicylic acid on physiological traits and parthenolide level of two feverfew (Tanacetum parthenium Linn. And Jelitto cv. Pharmasaat). Med Plants 17(4):68–82 (In Farsi)
Anjum SA, Xie XY, Wang LC, Saleem MF, Man C, Lei W (2011) Morphological, physiological and biochemical responses of plants to drought stress. African J Agr Res 6:2026–2032
Praba ML, Cairns J, Babu R, Lafitte H (2009) Identification of physiological traits underlying cultivar differences in drought tolerance in rice and wheat. J Agron Crop Sci 195:30–46
Tavallali V, Karimi S, Espargham O (2018) Boron enhances antioxidative defense in the leaves of salt-affected Pistacia vera seedlings. Hort J 87(1):55–62
Mirfattahi Z, Karimi S, Roozban MR (2017) Salinity induced changes in water relations, oxidative damage and morpho-physiological adaptations of pistachio genotypes in soilless culture. Acta Agr Slov 109(2):291–302
Sangwan NS, Farooqi Abad AH, Sangwan RS (1994) Effect of drought stress on growth and essential oil metabolism in lemongrasses. New Phytol 128:173–179
Lebaschi MH, Sharifi Ashurabadi A (2004) Growth indices of some species of medicinal plants under different drought stress conditions. Iranian J Med Aroma Plants Res 20(3):254–259 (In Farsi)
Salehi Arjmand H (2005) The effect of environmental stresses on the increase of secondary metabolites in plants. Proceedings of the National Conference on Sustainable Development of Medicinal Plants 307–305. (In Farsi)
Letchamo W, Marquard R, Gosselin A (1994) Effects of water supply and light intensity on growth and essential oil of two Thymus vulgaris selections. Angewandte Bot 68:83–88
Baher ZF, Mirza M, Ghorbanli M, Rezaii MB (2002) The influence of water stress on plant height, herbal and essential oil yield and composition in Satureja hortensis L. Flavour Frag J 17(4):275–277
Guntzer F, Keller C, Meunier JD (2012) Benefits of plant silicon for crops: a review. Agron Sustain Dev 32(1):201–213
Liang Y, Sun W, Zhu YG, Christie P (2007) Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: a review. Environ Pollu 147(2):422–428
Shen X, Zhou Y, Duan L, Li Z, Eneji AE (2010) Silicon effects on photosynthesis and antioxidant parameters of soybean seedlings under drought and ultraviolet-B radiation. J Plant Physiol 167(15):1248–1252
Marschner H (2011) Marschner’s mineral nutrition of higher plants. Academic press, University of adelaide, Australia
Waraich EA, Ahmad R, Ashraf MY (2011) Role of mineral nutrition in alleviation of drought stress in plants. Austr J Crop Sci 5(6):764
Epstein E (2001) Silicon in plants: facts vs. concepts. In Studies in Plant Science 8:1–15
Lux A, Luxová M, Abe J, Tanimoto E, Hattori T, Inanaga S (2003) The dynamics of silicon deposition in the sorghum root endodermis. New Phytol 158(3):437–441
Ma JF, Goto S, Tamai K, Ichii M (2001) Role of root hairs and lateral roots in silicon uptake by rice. Plant Physiol 127(4):1773–1780
Qin GZ, Tian SP (2009) Enhancement of biocontrol activity of Cryptococcus laurentii by silicon and the possible mechanisms involved. Phytopathol 95(1):69–75
Cooke J, Leishman MR (2011) Consistent alleviation of abiotic stress with silicon addition: a meta-analysis. Funct Ecol 30(8):1340–1357
Ouzounidou G, Giannakoula A, Ilias I, Zamanidis P (2016) Alleviation of drought and salinity stresses on growth, physiology, biochemistry and quality of two Cucumis sativus L. cultivars by Si application. Braz J Bot 39(2):531–539
Pei ZF, Ming DF, Liu D, Wan GL, Geng XX, Gong HJ, Zhou W (2010) Silicon improves the tolerance to water-deficit stress induced by polyethylene glycol in wheat (Triticum aestivum L.) seedlings. J Plant Growth Regul 29(1):106–115
Gong HJ, Chen KM, Chen GC, Wang SM, Zhang CL (2003) Effects of silicon on growth of wheat under drought. J Plant Nutr 26(5):1055–1063
Bao-Shan L, Shao-qi D, Chun-hui L, Li-jun F, Shu-chun Q, Min Y (2004) Effect of TMS (nanostructured silicon dioxide) on growth of Changbai larch seedlings. J Forest Res 15:138–140
Siddiqui MH, Al-Whaibi MH, Sakran AM, Ali HM, Basalah MO, Faisal M, Alatar A, Al-Amri AA (2013) Calcium-induced amelioration of boron toxicity in radish. J Plant Growth Regul 32:61–71
Li B, Tao G, Xie Y, Cai X (2012) Physiological effects under the condition of spraying nano-SiO2 onto the Indocalamus barbatus McClure leaves. J Nanjing Forest Uni (Natural Sciences Edition) 4:161–164
Yinfeng X, Bo L, Qianqian Z, Chunxia Z, Kouping L, Gongsheng T (2011) Effects of nano-TiO2 on photosynthetic characteristics of Indocalamus barbatus. J Northeast Forest Uni 39:22–25
Siddiqui MH, Al-Whaibi MH, Faisal M, Al Sahli AA (2014) Nanosilicon dioxide mitigates the adverse effects of salt stress on Cucurbita pepo L. Environ Toxicol Chem 33(11):2429–2437
Habibi G, Hajiboland R (2013) Alleviation of drought stress by silicon supplementation in pistachio (Pistacia vera L.) plants. Folia Hort 25(1): 21–29
Barrs H, Weatherley P (1962) A re-examination of the relative turgidity technique for estimating water deficits in leaves. Australia J Biol Sci 15(3):413–428
Sairam RK (1994) Effect of moisture stress on physiological activities of two contrasting wheat genotypes. Indian J Exp Biol 32:584–593
Alexieva V, Sergiev I, Mapelli S, Karanov E (2001) The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ 24:1337–1344
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Rastogi1 A, Tripathi DK, Yadav S, Chauhan DK, Živčák M, Ghorbanpour M, El-Sheery NI, Brestic M (2019) Application of silicon nanoparticles in agriculture. 3 Biotech 9(90):1–11
Hussain S, Mumtaz M, Manzoor S, Shuxian L, Ahmed I, Skalicky M, Brestic M, Rastogi A, Ulhassan Z, Shafiq I, Allakhverdiev SI, Khurshid H, Yang W, Liu W (2021) Foliar application of silicon improves growth of soybean by enhancing carbon metabolism under shading conditions. Plant Physiol Biochem 159:43–52
Timmusk S, El-Daim I, Copolovici L, Tanilas T, Kännaste A, Behers L, Nevo E, Seisenbaeva G, Stenström E, Niinemets Ü (2014) Drought-tolerance of wheat improved by rhizosphere bacteria from harsh environments: enhanced biomass production and reduced emissions of stress volatiles. PLoS ONE 9(5):96–86
Gong H, Chen K (2012) The regulatory role of silicon on water relations, photosynthetic gas exchange, and carboxylation activities of wheat leaves in field drought conditions. Acta Physiol Planta 34(4):1589–1594
Karimi S, Rahemi M, Rostami AA, Sedaghat S (2018) Drought effects on growth, water content and osmoprotectants in four olive cultivars with different drought tolerance. Int J Fruit Sci 18(3):254–267
Khan A, Bilal S, Khan AL, Imran M, Shahzad R, Al-Harrasi A (2020) Silicon and gibberellins: synergistic function in harnessing ABA signaling and heat stress tolerance in date palm (phoenix dactylifera L.). Plants (Basel, Switzerland) 9: 620.
Mirzaei Sh, Karimi S (2017) The use of iron nanoclates to improve the salinity tolerance of pistachio seedlings of Pistacia vera L. in alkaline calcareous medium. The first international conference on new technologies in science, Amol, Mazandaran, Iran, Amol University of New Technologies. (In Farsi)
Zhang J, Zou W, Li Y, Feng Y, Zhang H, Wu Z, Tu Y, Wang Y, Cai X, Peng L (2015) Silica distinctively affects cell wall features and lignocellulosic saccharification with large enhancement on biomass production in rice. Plant Sci 239:84–91
Mohamed MA, El-Sayed MA, El-Wahab HA (2015) Response of Succary mango trees to foliar application of silicon and boron. World Rural Observation 7(2):93–98
Ali A, Tahir M, Amin M, Basra SMA, Maqbool M, Lee D (2013) Si induced stress tolerance in wheat (Triticum aestivum L.) hydroponically grown under water deficit conditions. Bulg J Agr Sci 19(5):952–958
Karimi S, Karami H, Mokhtassi-Bidgoli A, Tavallali V, Vahdati K (2018) Inducing drought tolerance in greenhouse grown Juglans regia by imposing controlled salt stress: The role of osmotic adjustment. Sci Hort 239:181–192
Ma J, Takahashi E (1990) Effect of silicon on the growth and phosphorus uptake of rice. Plant Soil 126:115–119
Takahashi E, Ma J, Miyake Y (1990) The possibility of silicon as an essential element for higher plants. Comments Agr Food Chem 2:99–102
Wang J, Liu M, Li D (2009) Effects of silicon enrichment on photosynthetic characteristics and yield of strawberry. Northern Hort 12:90–92
Gao C, Liu J, Chang H, Yu X, Xu H (2013) Effects of silicon on rice leaf photosynthesis and ultrastructure. J Jilin Agr Uni 33:1–4
Quan R, Shang M, Zhang H, Zhao Y, Zhang, (2004) Improved chilling tolerance by transformation with beta gene for the enhancement of glycinebetaine synthesis in maize. Plant Sci 166:141–149
Sakamoto A, Murata N (2002) The role of glycine betaine in the protection of plants from stress: Clues from transgenic plants. Plant, Cell Environ 25:163–171
Rastogi A, Yadav S, Hussain S, Kataria S, Hajihashemi Sh, Kumari P, Yang X, Brestic M (2021) Does silicon really matter for the photosynthetic machinery in plants…? Plant Physiol Biochem 169:40–48
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Kim YH, Khan AL, Waqas M, Lee IJ (2017) Silicon regulates antioxidant activities of crop plants under abiotic-induced oxidative stress: a review. Front Plant Sci 8:510
Frew A, Weston LA, Reynolds OL, Gurr GM (2018) The role of silicon in plant biology: a paradigm shift in research approach. Annal bot 121:1265–1273
Rastogi A, Yadav DK, Szymanska R, Kruk J, Sedlarova M, Pospísil P (2014) Singlet oxygen scavenging activity of tocopherol and plastochromanol in Arabidopsis thaliana: relevance to photooxidative stress. Plant Cell Environ 32:392–401
Liang Y, Chen Q, Liu Q, Zhang W, Ding R (2003) Exogenous silicon (Si) increases antioxidant enzyme activity and reduces lipid peroxidation in roots of salt-stressed barley (Hordeum vulgare L.). J Plant Physiol 160:1157–1164
Moussa HR (2006) Influence of exogenous application of silicon on physiological response of salt-stressed maize (Zea mays L.). Int J Agric Biol 8:293–297
Malcovska SM, Ducaiova Z, Backor M (2014) Impact of silicon on maize seedlings exposed to short-term UV-B irradiation. Biologia 69:1349–1355
Karimi S, Tavallali V, Wirthensohn M (2017) Boron amendment improves water relations and performance of Pistacia vera under salt stress. Sci Hort 241:252–259
Jayawardana H, Weerahewa H, Saparamadu M (2014) Effect of root or foliar application of soluble silicon on plant growth, fruit quality and anthracnose development of capsicum. Tropic Agr Res 26(1):2–14
Karmollachaab A, Bakhshandeh A, Gharineh MH, Telavat MM, Fathi G (2013) Effect of silicon application on physiological characteristics and grain yield of wheat under drought stress condition. Int J Agron Plant Prod 4(1):30–37
Chen W, Yao X, Cai K, Chen, (2011) Silicon alleviates drought stress of rice plants by improving plant water status, photosynthesis and mineral nutrient absorption. Biol Trace Element Res 142(1):67–76
Petridis A, Therios I, Samouris G, Tananaki C (2012) Salinity-induced changes in phenolic compounds in leaves and roots of four olive cultivars (Olea europaea L.) and their relationship to antioxidant activity. Environ Exp Bot 79:37–43
Suriyaprabha R, Karunakaran G, Yuvakkumar R, Prabu P, Rajendran V, Kannan N (2012) Growth and physiological responses of maize (Zea mays L.) to porous silica nanoparticles in soil. J Nanopart Res 14:1294
Hura T, Hura K, Grzesiak S (2009) Possible contribution of cell-wall- bound ferulic acid in drought resistance and recovery in triticale seedlings. J Plant Physiol 166:1720–1733
Marete EN, Jacquier JC, O’Riordan D (2009) Effects of extraction temperature on the phenolic and parthenolide contents, and colour of aqueous feverfew (Tanacetum parthenium) extracts. Food chem 117(2):226–231
Mousavi H, Mehdi Nejad N, Fakhri B, MajdiM, Heydari F (2016) Effect of some nanoparticles on the expression of Jermacrene A synthase (TpGAS) and parthenolide synthase (TpPTS) genes in Tanacetum parthenium L. under dehydration stress. Iran Med Aroma Plants Res 36(2): 314–324 (In Farsi)
Ahmadian A, Ghanbari A, Siah Sar B, Heydari M, Ramroodi M, Mousavi Nik M (2010) Effect of chemical, animal and compostresidues on yield, yield components, some physiological characteristics and amount of feverfew essential oil under drought stress conditions. Iran Agr Res 8(4):668–676 (In Farsi)
Alaei S (2019) Essential oil Content and Composition of Dracocephalum Moldavica under Different Irrigation Regimes. Int J Hort Sci Tech 6(2):167–175
Munns R (1993) Physiological process limiting plant growth in saline soil: some dogmass and hypotheses. Plant Cell Environ 16:15–24
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Shabnam Esmaili, and Bahram Amiri. The first draft of the manuscript was written by Vahid Tavallali and all authors commented on previous versions of the manuscript.
Corresponding author
Ethics declarations
Statements and Declaration
This manuscript has not been published and is not under consideration for publication elsewhere. The authors declare that there are no conflicts of interest to disclose. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript. The authors have no relevant financial or non-financial interests to disclose.
Inform Consent
All the authors have informed consent.
Consent for Publication and Consent to Participate
All authors read and approved the final manuscript for publication in Silicon.
Research Involving Human and Animal Participants
This research not involved human participants and/or animals.
Conflict of Interest
This manuscript has no potential conflicts of interest (financial or non-financial).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Esmaili, S., Tavallali, V., Amiri, B. et al. Foliar Application of Nano-Silicon Complexes on Growth, Oxidative Damage and Bioactive Compounds of Feverfew Under Drought Stress. Silicon 14, 10245–10256 (2022). https://doi.org/10.1007/s12633-022-01754-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12633-022-01754-z