Abstract
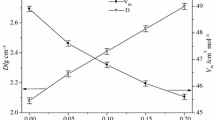
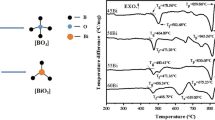
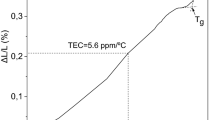
The glass formation region in the MgO–B2O3–SiO2 system was determined by the conventional melt-quenching technique at 1450 °C. The homogeneous transparent glasses were obtained in the central part of the magnesium borosilicate system in the region between 40 and 60 mol% MgO content. The amorphous and crystalline phases were examined by means of X-ray diffraction, differential thermal analysis, and Fourier-transform infrared spectroscopy. According to differential thermal analysis data, the glass transition temperature ranges between 645 and 705 °C, and the glass crystallization temperature varies between 830 and 985 °C. The thermal stability of glasses decreases with the increase of MgO content. Some physical characteristics (thermal expansion coefficient, density, molar volume, and volume resistivity) of the glass samples were estimated as well. The thermal expansion coefficient, density, and molar volume of the MgO–B2O3–SiO2 glasses obtained in this study ranged from 5.4 to 7.6 ppm/°С, 2.53 to 2.70 g/cm3, and 18.56 to 22.51 cm3/mol, respectively, depending on the glass composition. Fourier transform infrared spectroscopy results showed that the network of glasses consists mainly of BO3 and SiO4 structural units. These results may lead to the development of new materials with interesting applications in diverse fields because of their particular structural and physicochemical properties.
Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author on reasonable request.
References
Mahmoud K, Alsubaie A, Wahab EAA et al (2021) Research on the effects of yttrium on bismuth Titanate borosilicate glass system. Silicon. https://doi.org/10.1007/s12633-021-01125-0
Al-Baradi AM, Wahab EAA, Shaaban KS (2021) Preparation and characteristics of B2O3–SiO2–Bi2O3–TiO2–Y2O3 glasses and glass-ceramics. Silicon. https://doi.org/10.1007/s12633-021-01286-y
Shaaban KS, Al-Baradi AM, Wahab EAA (2021) The impact of Y2O3 on physical and optical characteristics, polarizability, optical basicity, and dispersion parameters of B2O3 – SiO2 – Bi2O3 – TiO2 glasses. Silicon. https://doi.org/10.1007/s12633-021-01309-8
Santha N, Shamsudeen S, Karunakaran NT, Isuhak Naseemabeevi J (2011) Spectroscopic, dielectric and optical properties of 60ZnO-30B2O3-10SiO2 glass-Al2O3 composites. Int J Appl Ceram Techno 8(5):1042–1049. https://doi.org/10.1111/j.1744-7402.2011.02667.x
Kirdsiri K, Rajaramakrishna R, Damdee B et al (2019) Influence of alkaline earth oxides on Eu3+ doped lithium borate glasses for photonic, laser and radiation detection material applications. Solid State Sci 89:57–66. https://doi.org/10.1016/j.solidstatesciences.2018.12.019
Rozenenkova VA, Solntsev SS, Mironova NA (2013) Glass ceramic electric insulation coatings for thick-film energy-saturated systems. Glas Ceram 70:269–272. https://doi.org/10.1007/s10717-013-9558-x
Frolenkov KY (2009) Heat-resistant glass-ceramic coatings protecting low-alloyed steels against high-temperature gas corrosion. Prot Met Phys Chem Surf 45:444–449. https://doi.org/10.1134/S2070205109040121
Karasik E, Marchan R, Gorulya N (2019) Protective heat resistant coating for Inconel 718 additively manufactured parts. Proceedings of the International Astronautical Congress, IAC. 2019-October:IAC-19_C2_4_8_x49241
Anjana PS, Salinigopal MS, Gopakumar N (2021) Glasses and glass-ceramics as sealants in solid oxide fuel cell applications. In: Dhoble SJ et al (eds) Energy materials: fundamentals to applications. Elsevier, Amsterdam. https://doi.org/10.1016/B978-0-12-823710-6.00012-1
Rodríguez-López S, Pascual MJ (2021) Sintering/crystallization and viscosity of sealing glass-ceramics. Crystals 11(7):737. https://doi.org/10.3390/cryst11070737
Salinigopal MS, Gopakumar N, Anjana PS (2020) Alkaline earth based borosilicate glasses as sealants in solid oxide fuel cell applications. Silicon 12:101–107. https://doi.org/10.1007/s12633-019-00103-x
Han L, Song J, Zhang Q et al (2018) Synthesis, structure and properties of MgO-Al2O3-SiO2-B2O3 transparent glass-ceramics. Silicon 10:2685–2693. https://doi.org/10.1007/s12633-018-9806-3
Zaichuk A, Amelina A, Kalishenko Y et al (2021) Aspects of development and properties of densely sintered of ultra-high-frequency radio-transparent ceramics of cordierite composition. J Korean Ceram Soc 58:483–494. https://doi.org/10.1007/s43207-021-00125-5
Solntsev SS (2011) High-temperature composite materials and coatings on the basis of glass and ceramics for aerospace technics. Russ J Gen Chem 81:992–1000. https://doi.org/10.1134/S1070363211050306
Došler U, Kržmanc MM, Suvorov D (2012) Phase evolution and microwave dielectric properties of MgO–B2O3–SiO2–based glass–ceramics. Ceram Int 38(2):61019–61025. https://doi.org/10.1016/j.ceramint.2011.08.025
Karasik EV, Hordieiev YS (2020) Calculation of thermal expansion, glass transition temperature and glass density in the system RO–Al2O3–B2O3–SiO2 (where RO=BaO, SrO, CaO, MgO, ZnO). Voprosy Khimii i Khimicheskoi Tekhnologii 6:69–74. https://doi.org/10.32434/0321-4095-2020-133-6-69-74
Youngman RE (2021) Borosilicate Glasses. In: Richet P et al (eds) Encyclopedia of glass science, technology, history, and culture. Wiley, New York. https://doi.org/10.1002/9781118801017.ch7.6
Shaaban KS, Wahab EAA, Shaaban ER et al (2020) Electronic polarizability, optical basicity, thermal, mechanical and optical investigations of (65B2O3–30Li2O–5Al2O3) glasses doped with Titanate. J Electron Mater 49:2040–2049. https://doi.org/10.1007/s11664-019-07889-x
Stolyarova VL et al (2010) Thermodynamic properties of silicate glasses and melts: VII. System MgO–B2O3–SiO2. Russ J Gen Chem 80(12):2405–2413. https://doi.org/10.1134/S1070363210120029
Hordieiev YS, Karasik EV, Amelina AA (2021) Properties of glasses in the system BaO–B2O3–SiO2–xAl2O3 (x=0; 5; 10 Mol %). Voprosy Khimii i Khimicheskoi Tekhnologii 3:83–89. https://doi.org/10.32434/0321-4095-2021-136-3-83-89
Hsiang HI, Chen CC, Yang SY (2019) Structure, crystallization, and dielectric properties of the Al2O3 filled CaO–B2O3–SiO2–Al2O3 glass composites for LTCC applications. Jpn J Appl Phys 58(9):091010. https://doi.org/10.7567/1347-4065/ab3cc5
Hordieiev YS, Amelina AA (2021) Glass formation and properties of glasses in the system SrO–B2O3–SiO2–xAl2O3 (x=0; 10 Mol.%). Voprosy Khimii i Khimicheskoi Tekhnologii 5:43–49. https://doi.org/10.32434/0321-4095-2021-138-5-43-49
Watts SJ, Hill RG, O’donnell MD, Law RV (2010) Influence of magnesia on the structure and properties of bioactive glasses. J Non-Cryst Solids 356(9–10):517–524. https://doi.org/10.1016/j.jnoncrysol.2009.04.074
Shahrabi S, Hesaraki S, Moemeni S, Khorami M (2011) Structural discrepancies and in vitro nanoapatite formation ability of sol–gel derived glasses doped with different bone stimulator ions. Ceram Int 37(7):2737–2746. https://doi.org/10.1016/j.ceramint.2011.04.025
Peng K, Fu L, Yang H, Ouyang J (2016) Perovskite LaFeO3/montmorillonite nanocomposites: synthesis, interface characteristics and enhanced photocatalytic activity. Sci Rep 6(1):1–10. https://doi.org/10.1038/srep19723
El Nahrawy AM, Abou Hammad AB, Mansour AM (2021) Compositional effects and optical properties of P2O5 doped magnesium silicate mesoporous thin films. Arab J Sci Eng 46:5893–5906. https://doi.org/10.1007/s13369-020-05067-4
Huang S, Li S, Wu F, Yue Y (2015) Effect of B2O3 on structure and properties of CaO–MgO–B2O3–Al2O3–SiO2 glasses. J Inorg Organomet Polym 25(4):816–822. https://doi.org/10.1007/s10904-015-0164-y
Luo W, Bao Z, Jiang W et al (2019) Effect of B2O3 on the crystallization, structure and properties of MgO–Al2O3–SiO2 glass-ceramics. Ceram Int 45(18):24750–24756. https://doi.org/10.1016/j.ceramint.2019.08.215
Gui H, Li C, Lin C et al (2019) Glass forming, crystallization, and physical properties of MgO-Al2O3-SiO2-B2O3 glass-ceramics modified by ZnO replacing MgO. J Eur Ceram Soc 39(4):1397–1410. https://doi.org/10.1016/j.jeurceramsoc.2018.10.002
El-Rehim AA, Zahran H, Yahia I et al (2021) Radiation, crystallization, and physical properties of cadmium borate glasses. Silicon 13:2289–2307. https://doi.org/10.1007/s12633-020-00798-3
Han J, Lai Y, Xiang Y et al (2017) Glass structure of the CaO–B2O3–SiO2–Al2O3–ZnO glasses system with different Si content. J Mater Sci Mater Electron 28(8):6131–6137. https://doi.org/10.1007/s10854-016-6291-6
Trégouët H, Caurant D, Majérus O et al (2017) Exploration of glass domain in the SiO2–B2O3–La2O3 system. J Non-Cryst Solids 476:158–172. https://doi.org/10.1016/j.jnoncrysol.2017.09.048
Acknowledgments
The authors gratefully acknowledge the financial support from the Ministry of Education and Science of Ukraine (project No. 0120 U101969).
Funding
This work was supported by the Ministry of Education and Science of Ukraine (project No. 0120 U101969).
Author information
Authors and Affiliations
Contributions
Hordieiev Yu.S. wrote the manuscript, conceived and designed the experiments, performed experimental work; Karasik E.V. assisted in experimental work; Zaichuk A.V. provided the reagents and assisted in statistical analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
The manuscript has not been published elsewhere and that it has not been submitted simultaneously for publication elsewhere.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare that they have no conflict of interest. The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hordieiev, Y.S., Karasik, E.V. & Zaichuk, A.V. Glass Formation in the MgO–B2O3–SiO2 System. Silicon 15, 1085–1091 (2023). https://doi.org/10.1007/s12633-022-01745-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12633-022-01745-0