Abstract
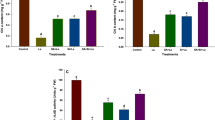
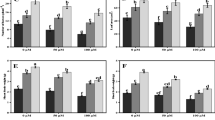
Silicon (Si) is very effective in the amelioration of heavy metal (HM) stress in different crop plants. This investigation was conducted to assess the protective role of Si in modulating aluminium (Al) uptake in 21 days old common buckwheat (Fagopyrum esculentum M.) plants. Exogenous Si (0.5 and 1 mM) treatments were used to study the effect on chlorophyll contents, anthocyanins, total phenolic content (TPC), total flavonoid content (TFC) and defence enzymes (phenylalanine ammonialyase (PAL), tyrosine ammonia lyase (TAL) and polyphenol oxidase (PPO) activities) against Al (0.2 and 0.4 mM) treatments. Results indicated that Al phytotoxicity negatively affected the above mentioned parameters while as Si application markedly reduced the phytotoxicity caused by Al3+ ions by improving their contents and increasing defense enzyme activities. Si thus alleviates phytotoxicity caused by Al ions and also promotes the production of phenolic and flavonoid compounds by stimulating the phenylpropanoid pathway. The present study revealed that exogenous Si application influenced the phenylpropanoid pathway in common buckwheat irrespective of being an Al tolerant plant thereby decreasing the oxidative damage and enhancing free radical scavenging activity by increasing the production of phenolic and flavonoid compounds under Al stress.
Similar content being viewed by others
Data Availability
The datasets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
Dar FA, Pirzadah TB, Tahir I, Rehman RU (2020) Deciphering the in-vitro antioxidant potential and mineral analysis of Fagopyrum species from Kashmir region. J Rep Pharm Sci 9(2):235–245
Bian M, Zhou M, Sun D, Li C (2013) Molecular approaches unravel the mechanism of acid soil tolerance in plants. Crop J 1(2):91–104
Singh D, Chauhan SK (2011) Organic acids of crop plants in aluminum detoxification. Curr Sci 100:1509–1515
Liu J, Pineros MA, Kochian LV (2014) The role of aluminum sensing and signaling in plant aluminum resistance. J Integr Plant Biol 56(3):221–230
Matsumoto H (2000) Cell biology of aluminum toxicity and tolerance in higher plants: review article. Int Rev Cytol 200:1–46
Shetty R, Vidya CSN, Prakash NB, Lux A, Vaculik M (2021) Aluminum toxicity in plants and its possible mitigation in acid soils by biochar: a review. Sci Total Environ 765:142744. https://doi.org/10.1016/j.scitotenv.2020.142744
Singh S, Tripathi DK, Singh S, Sharma S, Dubey NK, Chauhan DK, Vaculik M (2017) Toxicity of aluminum on various levels of plant cells and organism: a review. Environ Exp Bot 137:177–193
Sade H, Meriga B, Surapu V, Gadi J, Sunita MSL, Suravajhala P, Kishor PK (2016) Toxicity and tolerance of aluminum in plants: tailoring plants to suit to acid soils. Biometals 29:187–210
Vega I, Nikolic M, Pontigo S, Godoy K, de La Luz Mora M, Cartes P (2019) Silicon improves the production of high antioxidant or structural phenolic compounds in barley cultivars under aluminum stress. Agronomy 9:388. https://doi.org/10.3390/agronomy9070388
Emamverdian A, Ding Y, Xie Y, Sangari S (2018) Silicon mechanisms to ameliorate heavy metal stress in plants: A review. Hindawi Biomed Res. Int. 10 pages, article ID 8492898.
Epstein E (1994) The anomaly of silicon in plant biology. Proc Natl Acad Sci USA 91:11–17
Ma JF, Tamal K, Yamaji N, Mitani N, Konishi S, Katuhara M, Ishiguro M, Yano M (2006) A Si transporter in rice. Nature 440:688–691
Sommer M, Kaczorek D, Kuzyakov Y, Breuer J (2006) Silicon pools and fluxes in soils and landscapes: a review. J Plant Nutr Soil Sci 169:310–329
Exley C, Guerriero G, Lopez X (2020) How is silicic acid transported in plants? Silicon 12:2641–2645
Greger M, Landberg T, Vaculík M (2018) Silicon influences soil availability and accumulation of mineral nutrients in various plant species. Plants. 7:41. https://doi.org/10.3390/plants7020041
Etesami H, Jeong BR (2018) Silicon (Si): review and future prospects on the action mechanisms in alleviating biotic and abiotic stresses in plants. Ecotoxicol Environ Saf 147:881–896
Zargar SM, Mahajan R, Bhat JA, Nazir M, Deshmukh R (2019) Role of silicon in plant stress tolerance: opportunities to achieve a sustainable cropping system: review. 3 Biotech 9:73. https://doi.org/10.1007/s13205-019-1613-z
Boudet AM (2007) Evolution and current status of research in phenolic compounds. Phytochemistry 68:2722–2735
Hahlbrock K, Scheel D (1989) Physiology and molecular biology of phenylpropanoid metabolism. Annu Rev Plant Physiol Plant Mol Biol 40:347–369
Huang J, Gu M, Lai Z, Fan B, Shi K, Zhou YH, Yu JQ, Chen Z (2010) Functional analysis of the Arabidopsis PAL gene family in plant growth, development, and response to environmental stress. Plant Physiol 153:1526–1538
Koukol J, Conn EE (1961) The metabolism of aromatic compounds in higher plants. IV Purification and properties of the phenylalanine deaminase of Hordeum vulgare. J Biol Chem 236:2692–2698
Dixon RA, Paiva NL (1995) Stress-induced phenylpropanoid metabolism. Plant Cell 7:1085–1097. https://doi.org/10.1105/tpc.7.7.1085
Kim DS, Hwang BK (2014) An important role of the pepper phenylalanine ammonia lyase gene (PAL1) in salicylic acid-dependent signaling of the defense response to microbial pathogens. J Exp Bot 65(9):2295–2306
Vogt T (2010) Phenylpropanoid biosynthesis. Mol Plant 3:2–20
Singh S, Parihar P, Singh R, Singh VP, Prasad SM (2016) Heavy metal tolerance in plants: role of transcriptomics, proteomics, metabolomics, and ionomics. Front Plant Sci 6:1143. https://doi.org/10.3389/fpls.2015.01143
Smirnov OE, Kosyan AM, Kosyk OI, Taran NY (2015) Response of phenolic metabolism induced by aluminum toxicity in Fagopyrumesculentum Moench. Plants. Ukr Biochem J 87(6):129–135
Rice-Evans C, Miller N, Paganga G (1997) Antioxidant properties of phenolic compounds: review. Trends Plant Sci 2(4):152–159
Jahnen W, Hahlbrock K (1988) Differential regulation and tissue-specific distribution of enzymes of phenylpropanoid pathways in developing parsley seedlings. Planta 173:453–458
Jun M, Fu HY, Hong J, Wan X, Yang CS, Ho CT (2003) Comparison of antioxidant activities of isoflavones from kudzu root (Pueraria lobata Ohwi). J Food Sci 68:2117–2122. https://doi.org/10.1111/j.1365-2621.2003.tb07029.x
Winke-Shirley B (2002) Biosynthesis of flavonoids and effects of stress. Plant Biol 5:218–223
Hura T, Grzesiak S, Hura K, Thiemt E, Tokarz K, Wedzomy M (2007) Physiological and biochemical tools useful in drought-tolerance detection in genotypes of winter triticale: accumulation of ferulic acid correlates with drought tolerance. Ann Bot 100:767–775
Hura T, Hura K, Grzesia S (2008) Contents of total phenolics and ferulic acid and PAL activity during water potential changes in leaves of maize single-cross hybrids of different drought tolerance. J Agron Crop Sci 194:104–112
Nadernejad N, Ahmadimoghadam A, Hosseinifard J, Pourseyedi S (2012) Phenylalanine ammonia-lyase activity, total phenolic and flavonoid content in flowers, leaves, hulls and kernels of three pistachio (Pistacia vera L.) cultivars. Am Eurasian J Agric Environ Sci 12(6):807–814
Korkina LG (2007) Phenylpropanoid as naturally occurring antioxidant from plant defense to human health. Cell Mol Biol 53:15–25
Rosler J, Krekel F, Amrhein N, Jurg S (1997) Maize phenylalanine ammonia-lyase has tyrosine ammonia-lyase activity. Plant Physiol 113:175–179
Solecka D (1997) Role phenylpropanoid compounds in plant response to different stress factor. Acta Physiol Plant 19:257–268
Wajahatullah K, Balkrishnan P, Donald LS (2002) Chitosan and chitin oligomers increase phenylalanine ammonia-lyase and tyrosine ammonia-lyase activities in soybean leaves. Plant Physiol 160:859–863
Hoagland DR, Arnon DI (1950) The water-culture for growing plants without soil. Calif Agric Ext Serv Circ 347:32
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic membranes. Methods Enzymol 148:350–382
Duxbury AC, Yentsch CS (1956) Plankton pigment monographs. J Mar Res 15:91–101
Mancinelli AL, Huang-Yang CP, Lindquist P, Anderson R, Rabino I (1975) Photocontrol of anthocyanin synthesis. The action of streptomycin on the synthesis of chlorophyll and anthocyanin. Plant Physiol 55:251–257
Singelton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungistic acid reagents. Am J EnolVitic 16:144–158
Velioglu YS, Mazza G, Gao L, Oomah BD (1998) Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J Agric Food Chem 46:4113–4117
Zhishen J, Mengcheng T, Jianming W (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64:555–559
Gonzalez-Aguilar GA, Tizuado-Hernandoz ME, Zavaleeta-Gatica R, Martinez-Tellez MA (2004) Methyl jasmonate treatments reduce chilling injury and activate the defense response of guava fruits. Biochem Biophys Res Commun 313:694–701
Liu H, Jiang W, Bi Y, Luo Y (2005) Postharvest BTH treatment induces resistance of peach (Prunus persica L. cv. Jiubao) fruit to infection by Penicillium expansum and enhances activity of fruit defense mechanisms. Postharvest Biol Technol 35:263–269
Pinto SDS, de Souza AE, Oliva MA, Pereira EG (2016) Oxidative damage and photosynthetic impairment in tropical rice cultivars upon exposure to excess iron. Sci Agric 73(3):217–226
Yruela I (2013) Transition metals in plant photosynthesis. Metallomics 5(9):1090–1109
Asgher M, Per TS, Anjum S, Khan MIR, Masood A, Verma S, Khan NA (2017) Contribution of glutathione in heavy metal stress tolerance in plants. In: Khan M, Khan N (eds) Reactive oxygen species and antioxidant Systems in Plants: role and regulation under abiotic stress. Springer, Singapore, pp 297–313
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions [Review]. Journal of Botany, 2012, 26 pages, article ID 217037. https://doi.org/10.1155/2012/217037
Pinheiro JN, Marques C, Pinto G, Mestiri A, Mendo S, Gomes NC, Goncalves F, Rocha Santos T, Duarte AC, Rombke J, Sousa JP, Ksibi M, Haddioui A, Pereira R (2013) The performance of Fraxinus angustifolia as a helper for metal phytoremediation programs and its relation to the endophytic bacterial communities. Geoderma 202(203):171–182
Miyasaka SC, Hue NV, Dunn MA (2007) Aluminum-Handbook of plant nutrition. In: Allen DJP, Barker V (eds) Handbook of plant nutrition (pp. 439–498). CRC Press
Asada K (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 141(2):391–396
Farooq MA, Ali S, Hameed A, Ishaque W, Mahmood K, Iqbal Z (2013) Alleviation of cadmium toxicity by silicon is related to elevated photosynthesis, antioxidant enzymes; suppressed cadmium uptake and oxidative stress in cotton. Ecotoxicol Environ Saf 96:242–249
Imtiaz M, Rizwan MS, Mushtaq MA, Ashraf M, Shahzad SM, Yousaf B, Saeed DA, Rizwan M, Nawaz MA, Mehmood S (2016) Silicon occurrence, uptake, transport and mechanisms of heavy metals, minerals and salinity enhanced tolerance in plants with future prospects: a review. J Environ Manag 183:521–529
Rizwan M, Meunier JD, Miche H, Keller C (2012) Effect of silicon on reducing cadmium toxicity in durum wheat (Triticum turgidum L. cv. Claudio W.) grown in a soil with aged contamination. J Hazard Mater 209-210:326–334
Tripathi DK, Singh VP, Prasad SM, Chauhan DK, Dubey NK, Rai AK (2015) Silicon-mediated alleviation of Cr (VI) toxicity in wheat seedlings as evidenced by chlorophyll fluorescence, laser induced breakdown spectroscopy and anatomical changes. Ecotoxicol Environ Saf 113:133–144. https://doi.org/10.1016/j.ecoenv.2014.09.029
Ali S, Farooq MA, Yasmeen T, Hussain S, Arif MS, Abbas F, Bharwana SA, Zhang G (2013) The influence of silicon on barley growth, photosynthesis and ultra-structure under chromium stress. Ecotoxicol Environ Saf 89:66–72
Doncheva SN, Poschenrieder C, Stoyanova ZI, Georgieva K, Velichkova M, Barcelo J (2009) Silicon amelioration of manganese toxicity in Mn-sensitive and Mn-tolerant maize varieties. Environ Exp Bot 65:169–197
Feng J, Shi Q, Wang X, Wei M, Yang F, Xu H (2010) Silicon supplementation ameliorated the inhibition of photosynthesis and nitrate metabolism by cadmium (cd) toxicity in Cucumis sativus L. Sci Hortic 123:521–530
Song A, Li P, Fan FL, Li ZJ, Liang YC (2014) The effect of silicon on photosynthesis and expression of its relevant genes in rice (Oryza sativa L.) under high-zinc stress. PLoS One 9:1–21
Vatehova Z, Kollarova K, Zelko I, Richterova-Kucerova D, Bujdos M, Liskova D (2012) Interaction of silicon and cadmium in Brassica juncea and Brassica napus. Biologia. 67(3):498–504
Ali S, Rizwan M, Ullah N, Bharwana SA, Waseem M, Farooq MA, Abbasi GH, Farid M (2016) Physiological and biochemical mechanisms of silicon-induced copper stress tolerance in cotton (Gossypium hirsutum L.). Acta Physiol Plant 38:262
Dorneles AOS, Pereira AS, Sasso VM, Possebom G, Tarouco CP, Schorr MRW, Rossato L, Ferreira PAA, Tabaldi LA (2019) Aluminum stress tolerance in potato genotypes grown with silicon. Bragantia 78(1):12–25
Schutzendubel A, Polle A (2002) Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J Exp Bot 53(372):1351–1365
Collin B, Doelsch E, Keller C, Cazevieille P, Tella M, Chaurand P, Panfili F, Hazemann JL, Meunier JD (2014) Copper distribution and speciation in bamboo exposed to a high cu concentration and Si supplementation. First evidence on the presence of reduced copper bound to sulfur compounds in Poaceae. Environ Pollut 187:22–30
Wang Y, Stass A, Horst WJ (2004) Apoplastic binding of aluminum is involved in silicon-induced amelioration of aluminum toxicity in maize. Plant Physiol 136:3762–3770
Fleck AT, Nye T, Repenning C, Stahl F, Zahn M, Schenk MK (2011) Silicon enhances suberization and lignification in roots of rice (Oryza sativa). J Exp Bot 62:2001–2011
Fleck AT, Schulze S, Hinrichs M, Specht A, Wassmann F, Schreiber L, Schenk MK (2015) Silicon promotes exodermal casparian band formation in Si accumulating and Si-excluding species by forming phenol complexes. PLoS One 10:e0138555
Pontigo S, Godoy K, Jimenez H, Gutierrez-Moraga A, Mora ML, Cartes P (2017) Silicon-mediated alleviation of aluminum toxicity by modulation of Al/Si uptake and antioxidant performance in ryegrass plants. Front Plant Sci 8:642. https://doi.org/10.3389/fpls.2017.00642
Song A, Xue G, Cui P, Fan F, Liu H, Yin C, Sun W, Liang Y (2016) The role of silicon in enhancing resistance to bacterial blight of hydroponic- and soil-cultured rice. Sci Rep 6:24640. https://doi.org/10.1038/srep24640
Kidd PS, Llugany M, Poschenrieder CH, Gunsé B, Barceló J. (2001). The roleof root exudates in aluminium resistance and silicon-induced amelioration of aluminium toxicity in three varieties of maize (Zea mays L.). J Exp Bot 52(359):1339-1352.
Diaz J, Bernal A, Pomar F, Merino F (2001) Induction of shikimate dehydrogenase and peroxidase in pepper (Capsicum annuum L.) seedlings in response to copper stress and its relation to lignification. Plant Sci 161:179–188
Saha D, Mandal S, Saha A (2012) Copper induced oxidative stress in tea (Camellia sinensis) leaves. J Environ Biol 33(5):861–866
Rogalla H, Romeheld V (2002) Effects of silicon on the availability of boron: possible effects on the phenol pathway and on the redox status in Cucumis sativus L. In: Goldbach HE, Rerkasem B, Wimmer MA, Brown PH, Thellier M, Bell RW (eds) Boron in plant and animal nutrition. Kluwer Academic/Plenum, New York, pp 205–213
Sharma A, Shahzad B, Rehman A, Bhardwaj R, Landi M, Zheng B (2019) Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress: review. Molecules 24:2452. https://doi.org/10.3390/molecules24132452
Araji S, Grammer TA, Gertzen R, Anderson SD, Mikulic-Petkovsek M, Veberic R, Phu ML, Solar A, Leslie CA, Dandekar AM, Escobar MA (2014) Novel roles for the polyphenol oxidase enzyme in secondary metabolism and the regulation of cell death in walnut (Juglans regia). Plant Physiol 164:1191–1203
Kapoor D, Kaur S, Bhardwaj R (2014) Physiological and biochemical changes in Brassica juncea plants under Cd-induced stress. Hindawi Publishing Corporation, BioMed Research International, V, 13 pages, article ID 726070.
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Sarvajeet SG, Narendra T (2010) Reactive oxygen species and antioxidant machinery in a biotic stress tolerance in crop plants. Annu Rev Plant Physiol 3:1–22
Li J, Leisner SM, Frantz J (2008) Alleviation of copper toxicity in Arabidopsis thaliana by silicon addition to hydroponic solutions. J Amer Soc Hort Sci 133(5):670–677
Ma JF, Chen ZC, Shen RF (2014) Molecular mechanisms of Al tolerance in gramineous plants. Plant Soil 381:1–12
Ferdinando M, Brunetti C, Fini A, Tattini M (2012) Flavonoids as antioxidants in plants under abiotic stresses. In: Ahmad P, Prasad MNV (eds) Abiotic stress responses in plants: metabolism, productivity and sustainability. Springer Science + Business Media, LLC, New York, pp 159–179
Acknowledgements
We are grateful to the Department of Bioresources, University of Kashmir, Hazratbal, Srinagar for allowing us to conduct this research and provides us necessary support in terms of providing logistic and financial assistance.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
FAD, IT and RUR conceived the problem, set up the experimental design and contributed to the writing, reviewing and final preparation of the manuscript. FAD conducted all the experiments, took readings and also performed the data analysis using software programs. KRH has critically analyzed the results and revised the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
The consent was obtained from individual or guardian participants.
Consent for Publication
That all authors have checked the manuscript and have agreed to the submission.
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dar, F.A., Tahir, I., Hakeem, K.R. et al. Silicon Application Enhances the Photosynthetic Pigments and Phenolic/Flavonoid Content by Modulating the Phenylpropanoid Pathway in Common Buckwheat under Aluminium Stress. Silicon 14, 323–334 (2022). https://doi.org/10.1007/s12633-021-01501-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12633-021-01501-w