Abstract
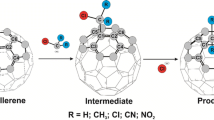
A density functional theory study was performed to design new N-heterocyclic silyl pincer fullerenes based on the reactions of diaminofullerene with chlorosilanes SiHRCl2. Reaction energies of the formation of pincer fullerene ligands increased through the substitution of flanking arms with CH3 and phenyl groups. However, substituting hydrogen of SiH2 with methyl slightly increased the corresponding reaction energies; replacing hydrogen with phenyl group decreased the reaction energies of the considered pincer fullerenes. While the calculated electrophilicity values of the pincer fullerenes are larger than the electrophilicity values obtained for the fullerene derivatives, the substitution of hydrogen atoms of central SiH2 and PH2 did not has a noticeable impact on the electrophilicity values of the pincer fullerenes. The only exception was SiHPh(NCH2PH2)2C60. Natural bonding orbital analysis showed that the delocalization of electrons from the lone pairs of phosphorous atoms to the n* orbital of transition metal atoms was a key factor for stabilizing the considered complexes. The strongest interaction was due to the delocalization of electrons from lone pairs of phosphorous atoms in the flanking arms to the LP* of transition metals, which was followed by the delocalization of electrons of the Si-H σ* orbitals to the LP* of transition metals.
Similar content being viewed by others
Data Availability
All data are provided in supplementary material.
References
Moulton CJ, Shaw BL (1976) Transition metal–carbon bonds. Part XLII. Complexes of nickel, palladium, platinum, rhodium and iridium with the tridentate ligand 2,6-bis[(di-t-butylphosphino)methyl]phenyl. J Chem Soc Dalton Trans:1020–1024
Asaya M, Morales-Morales D (2015) Non-symmetric pincer ligands: complexes and applications in catalysis. Dalton Trans 44:17432–17447
Wanniarachchi S, Liddle BJ, Toussaint J, Lindeman SV, Bennett B, Gardinier JR (2010) Chemical switching behaviour of tricarbonylrhenium(i) complexes of a new redox active ‘Pincer’ ligand. Dalton Trans 39:3167–3169
Prokhorov AM, Hofbeck T, Czerwieniec R, Suleymanova AF, Kozhevnikov DN, Yersin H (2014) Brightly luminescent Pt (II) pincer complexes with a sterically demanding carboranyl-phenylpyridine ligand: a new material class for diverse optoelectronic applications. J Am Chem Soc 136:9637–9642
Lam ES-H, Lam WH, Yam VW-W (2015) A study on the effect of dianionic tridentate ligands on the radiative and nonradiative processes for gold(III) alkynyl systems by a computational approach. Inorg Chem 54:3624–3630
Albrecht M, van Koten G (2001) Platinum group organometallics based on “Pincer” complexes: sensors, switches, and catalysts in memory of prof. Dr. Luigi M. Venanzi and his pioneering work in organometallic chemistry, particularly in PCP pincer chemistry. Angew Chem Int Ed 40:3750–3781
Peris E, Crabtree RH (2018) Key factors in pincer ligand design. Chem Soc Rev 47:1959–1968
Choi J, MacArthur AHR, Brookhart M, Goldman AS (2011) Dehydrogenation and related reactions catalyzed by iridium pincer complexes. Chem Rev 111:1761–1779
van Koten G, Milstein D (2013) Organometallic pincer chemistry. Springer-Verlag, Berlin-Heidelberg
Szabo KJ, Wendt OF (2014) Pincer and pincer-type complexes: applications in organic synthesis and catalysis. Wiley-VCH, Germany
Gupta M, Hagen C, Flesher RJ, Kaska WC, Jensen CM (1996) A highly active alkane dehydrogenation catalyst: stabilization of dihydrido rhodium and iridium complexes by a P–C–P pincer ligand. Chem Commun 2083-2084
Lawrence MAW, Green K-A, Nelson PN, Lorraine SC (2018) Review: pincer ligands-tunable, versatile and applicable. Polyhedron. 143:11–27
van der Vlugt JI, Reek JNH (2009) Neutral tridentate PNP ligands and their hybrid analogues: versatile non-innocent scaffolds for homogeneous catalysis. Angew Chem Int Ed 48:8832–8846
Stobart SR, Zhou X, Cea-Olivares R, Toscano A (2001) Activation of water and of Dioxygen by a Bis(diphenylphosphinopropyl)silyl (biPSi) complex of ruthenium(II): formation of Bis(diphenylphosphinopropyl)siloxo cage complexes. Concomitant oxygen atom insertion into a silicon−carbon bond. Organometallics 20:4766–4768
Tilley TD, Sangtrirutnugul P (2008) Alkyl and hydrido complexes of platinum(IV) supported by the Bis(8-quinolyl)methylsilyl ligand. Organometallics 27:2223–2230
Kwok W-H, Lu G-L, Rickard CEF, Roper WR, Wright LJ (2004) Nucleophilic substitution reactions at the Si–Cl bonds of the dichloro(methyl)silyl ligand in five- and six-coordinate complexes of ruthenium(II) and osmium(II). J Organomet Chem 689:2511–2522
Ruddy AJ, Mitton SJ, McDonald R, Turculet L (2012) ‘Hemilabile’ silyl pincer ligation: platinum group PSiN complexes and triple C–H activation to form a (PSiC)Ru carbene complex. Chem Commun 48:1159–1161
Korchin EE, Leitus G, Shimon LJW, Konstantinovski L, Milstein D (2008) Silanol-based pincer Pt(II) complexes: synthesis, structure, and unusual reactivity. Inorg Chem 47:7177–7189
Hill AF, Neumann H, Wagler J (2010) Bis(methimazolyl)silyl complexes of ruthenium. Organometallics 29:1026–1031
Dixon LSH, Hill AF, Sinha A, Ward JS (2014) N-heterocyclic silyl pincer ligands. Organometallics 33:653–658
Hu X, Jiang Z, Jia Z, Huang S, Yang X, Li Y, Gan L, Zhang S, Zhu D (2007) Amination of [60]fullerene by ammonia and by primary and secondary aliphatic amines - preparation of amino[60]fullerene peroxides. Chem Eur J 13:1129–1141
Janaki J, Premila M, Gopalan P, Sastry VS, Sundar CS (2000) Thermal stability of a fullerene-amine adduct. Thermochim Acta 356:109–116
Glen Miller P (2006) Reactions between aliphatic amines and [60]fullerene: a review. C R Chimie 9:952–959
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Account 120:215–241
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19:553–566
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su SJ, Windus TL, Dupuis M, Montgomery JA (1993) General atomic and molecular electronic structure system. J Comput Chem 14:1347–1363
Gordon MS, Schmidt MW (2005) In: Dykstra CE, Frenking G, Kim KS, Scuseria GE (eds) Advances in electronic structure theory: GAMESS a decade later in “theory and applications of computational chemistry: the first forty years” Elsevier, Amsterdam
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev 88:899–926
Reed AE, Weinstock RB, Weinhold F (1985) Natural population analysis. J Chem Phys 83:735–746
Hedberg K, Hedberg L, Bethune DS, Brown CA, Dorn HC, Johnson RD, Vries M (1991) Bond lengths in free molecules of buckminsterfullerene, C60, from gas-phase electron diffraction. Science 254:410–412
Hawkins JM, Meyer A, Lewis TA, Loren SD, Hollander FJ (1991) Crystal structure of osmylated C60: confirmation of the soccer ball framework. Science 252:312–313
Parr RG, Szentpály LV, Liu S (1999) Electrophilicity index. J Am Chem Soc 121:1922–1924
Parr RG, Donnelly RA, Levy M, Palke WE (1978) Electronegativity: the density functional viewpoint. J Chem Phys 68:3801–3807
Politzer P, Murry JS (2006) A link between the ionization energy ratios of an atom and its electronegativity and hardness. Chem Phys Lett 431:195–198
Acknowledgements
We gratefully acknowledge for the financial support from the Research Council of Alzahra University.
Code availability
Not applicable.
Author information
Authors and Affiliations
Contributions
Not applicable.
Corresponding author
Ethics declarations
Ethics Approval
All authors approved Ethics.
Consent to Participate
All the authors approve consent for participate of this submission.
Consent for Publication
All the authors approve consent for publication of this submission.
Conflicts of Interest/Competing Interests
There is no conflict of interest in the manuscript.
Additional Declarations for Articles in Life Science Journals that Report the Results of Studies Involving Humans and/or Animals
Not applicable
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 794 kb)
Rights and permissions
About this article
Cite this article
Anafcheh, M., Zahedi, M. Computational Design of New N-Heterocyclic Silyl Pincer Fullerenes. Silicon 14, 3871–3878 (2022). https://doi.org/10.1007/s12633-021-01168-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12633-021-01168-3