Abstract
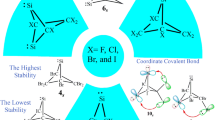
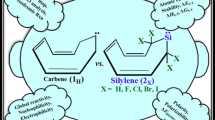
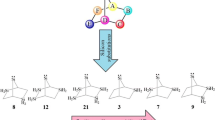
The density functional theory (DFT) calculations are carried out to assay the effects of nitrogen atoms on the stability and reactivity of singlet (s) and triplet (t) forms of novel silylenes with one, two, and three silylene centers (1s-18s and 1t-18t, receptively) at B3LYP/6-311 + + G** level of theory. Every one of the 36 silylenes scrutinized appears as minimum on its energy surface, for showing no negative force constant. All of our silylenes have singlets ground state and the highest stability belongs to silylene 2 (ΔEs−t = 44.71.95 kcal/mol). This clearly demonstrates the effect of intermolecular interactions (\(\sigma\)(C(E and G)−H(exo))→LP*S̈i). The aim of the present work was to consider the influence of nitrogen substituents on the stability (ΔEs−t), band gap (ΔΕHOMO−LUMO), nucleophilicity (N), electrophilicity (ω), and isodesmic reactions. Finally, our investigation offers new insights into the chemistry of novel bicyclic silylenes that can be applied as cumulated multi-dentate ligands.
Graphical Abstract
We have compared and contrasted the substituent effects on the stability and reactivity of singlet (s) and triplet (t) forms of novel silylenes with one, two, and three silylene centers (1s-18s and 1t-18t, receptively) at B3LYP/6-311 + + G** level of theory. All novel silylenes show singlet ground state and the highest stability belongs to silylene 2 (ΔEs−t = 44.71.95 kcal/mol). This stability can be related to its intermolecular interactions. The nonbonding electrons at the nitrogens (E or G) of silylenes 3s, 5s, 6s, 7s, 8s, 9s, 11s, 14s and 15s appear to have a tendency to interact with the empty p orbital of the silylene center and form a σ-bond.

Similar content being viewed by others
Data Availability
Not applicable
References
Kassaee MZ, Zandi H, Haerizade BN, Ghambarian M (2012) Comput Theor Chem 1001:39–43
Momeni MR, Shakib FA (2011) Organometallics 30(18):5027–5032
Koohi M, Bastami H (2020) Monatsh Chem-Chem Mon 151(1):11–23
Abedini N, Kassaee MZ, Cummings PT (2020) Silicon :1–7. https://doi.org/10.1007/s12633-020-00745-2
Mizuhata Y, Sasamori T, Tokitoh N (2009) Chem Rev 109:3479–3511
Bourissou D, Guerret O, Gabbai FP, Bertrand G (2000) Chem Rev 100:39–92
Barden CJ, Schaefer HF (2000) J Chem Phys 112:6515–6516
Lee EPF, Dyke JM, Wright TG (2000) Chem Phys Lett 326:143–150
Bruce M (1991) Chem Rev 91:197–257
Nefedov OM, Egorov MP, Ioffe AI, Menchikov LG, Zuev PS, Minkin VI, Simkin BY, Glukhovstev MN (1992) Pure Appl Chem 64:265–314
Schwartz RL, Davico GE, Ramond TM, Lineberger WC (1999) J Phys Chem A 103:8213–8221
Heaven MW, Metha GF, Buntine MA (2001) J Phys Chem A 105:1185–1196
Zachariah MR, Tsang W (1995) J Phys Chem 99:5308–5318
Boudjouk P, Black E, Kumarathasan R (1991) Organometal 10:2095–2096
Lucas DJ, Curtiss LA, Pople JA (1993) J Chem Phys 99:6697–6703
Cote DR, Van Nguyen S, Stamper AK, Armbrust DS, Tobben D, Conti RA, Lee GY (1999) IBM J Res Dev 43:5–38
Kassaee MZ, Buazar F, Soleimani-Amiri S (2008) J Mol Struct THEOCHEM 866:52–57
Kassaee MZ, Najafi Z, Shakib FA, Momeni MR (2011) J Organometal Chem 696:2059–2064
Schoeller WW, Eisner D (2004) Inorg Chem 43:2585–2589
Kirilchuk AA, Rozhenko AB, Leszczynski J (2017) Comp Theor Chem 1103:83–91
Nyulaszi L, Belghazi A, Kis-Szetsi S, Veszpremi T, Heinicke J (1994) Theochem 313:73–81
Zhou YP, Zh. Mo MPh, Luecke M, Driess (2018) Chem Eur J 24:4780–4784
Brück A, Gallego D, Wang W, Irran E, Driess M, Hartwig JF (2012) Angew Chem Int Ed 51:11478–11482
Ren H, Zhou YP, Bai Y, Cui C, Driess M (2017) Chem Eur J 23:5663–5667
Zhou YP, Wang Y, Driess M (2017) J Organometal Chem 829:2–10
Schmidt M, Blom B, Szilvasi T, Schomacker R, Driess M (2017) Eur J Inorg Chem 9:1284–1291
Mark JE, Allcock HR, West R (1992) Inorganic Polymers. Prentice Hall, New Jersey
Wisian-Neilson P, Allcock HR, Wynne KJ (1994) Inorganic and Organometallic Polymers II. ACS, Washington DC
Manners I (1996) Angew Chem Int Ed Eng 35:1602
Silaghi-Dumitrescu I, Haiduc I, Sowerby DB (1993) Inorg Chem 32(17):3755–3758
Strout DL (2001) J Phys Chem A 105(1):261–263
Strout DL (2000) J Phys Chem A 104(15):3364–3366
Silaghi-Dumitrescu I, Lara-Ochoa F, Bishof P, Haiduc I (1996) J Mol Struct Theochem 367:47–54
Camp D, Campitelli M, Hanson GR, Jenkins ID (2012) J Am Chem Soc 134:16188–16196
Nagase S (1995) Acc Chem Res 28(11):469–476
Soleimani Purlak N, Kassaee MZ (2020) J Phys Org Chem 33(6):e4053
Becke AD (1993) J Chem Phys 98:5648–5652
Yan Z, Truhlar DG (2008) Theor Chem Account 120:215–241
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su S, Windus TL, Dupuis M, Montgomery JA (1993) J Comput Chem 14:1347–1363
Adamo C, di Matteo A (1999) Adv Quantum Chem 36:45–75
Zhao Y, Truhlar DG (2008) Acc Chem Res 41(2):157–167
Abedini N, Kassaee MZ. Struct Chem :1–8. https://doi.org/10.1007/s11224-020-01715-5
Becke AD (1996) J Chem Phys 104:1040–1046
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Kassaee MZ, Ashenagar S (2018) J Mol Model 24:1–11
Domingo LR, Chamorro E, Perez P (2008) J Org Chem 73:4615–4624
Parr RG, Szentpaly L, Liu S (1999) J Am Chem Soc 121:1922–1924
Parr RG, Pearson RG (1983) J Am Chem Soc 105:7512–7516
Acknowledgements
The support from Tarbiat Modares University (TMU) is gratefully acknowledged.
Funding
Tarbiat Modares University
Author information
Authors and Affiliations
Contributions
Nastaran Abedini and Mohamad Z. Kassaee
Corresponding author
Ethics declarations
Not applicable
Conflicts of Interest
There are no conflicts to declare.
Consent to Participate
We have consent to participate.
Consent for Publication
We consent for publication
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOC 216 KB)
Rights and permissions
About this article
Cite this article
Abedini, N., Kassaee, M.Z. Substituent Effect On Structure, Stability, and Electronic Properties of the Novel Bicyclic Silylenes at DFT. Silicon 14, 2089–2095 (2022). https://doi.org/10.1007/s12633-021-00998-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12633-021-00998-5