Abstract
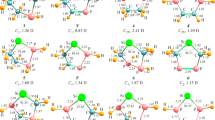
We have scrutinized thirteen new derivatives of cyclic and acyclic silylenes and compared their structural and thermodynamic parameters, at M06-2X/6–311++G** level of theory. The cyclic three- and five-membered silylenes include (2-hydroxy)cyclopropasilylene-2-ene (1), (2,3-dihydroxy)cyclopropasilylene-2-ene (2), (2-hydroxy)cyclopentasilylene-2,4-diene (3), and (2,5-dihydroxy)cyclopentasilylene-2,4-diene (4). The acyclic isomers consist of hydroxypropa-2-silylene (1′), (1,3-dihydroxy)propa-2-silylene (2′), (2-hydroxy)penta-3-silylene (3′), (2-hydroxy)penta-3-silylene-1,4-diene (3″), (2,4-dihydroxy)penta-3-silylene (4′), and (2,4-dihydroxy)penta-3-silylene-1,4-diene (4″). In addition, keto forms of 3″ (3”K) and 4″ (4”K1 and 4”K2) along with protonated forms of silylenes (1H, 1’H, 2H, 2’H, 3H, 3’H, 3”H, 3”K-H, 4H, 4’H, 4”H, 4”K1-H, and 4”K2-H) are investigated for determining their proton affinities (PAs) and intramolecular hydrogen bondings (IHBs). The results show that 4′ shows the lowest singlet-triplet energy gap (ΔEs-t = −19.03 eV) and band gap (ΔEH-L = −2.01 eV) and the highest nucleophilicity (N = 4.02 eV), chemical potential (μ = −3.38 eV), and PA (382.85 kcal/mol) which correlates with its strongest IHB. Atoms in molecules (AIM) analysis represents the highest electron density (ρ(r) = 0.033) at bond critical point (BCP) of IHB in 4′. The natural bond orbital (NBO) analysis shows the highest value of second-order perturbation stabilization energy (E2 = 7.85 kcal/mol) for 4′ which is in consistent with the lowest bond length of IHB (1.84 Å). Furthermore, the infrared (IR) spectroscopy indicates the lowest vibrational frequency of O-H bond νOH(νOH= 3542.87 cm−1) which verifies the strong IHB of 4′. The overall order of the IHB strength is 4′ > 4” > 4’H > 4”H > 2’ > 2’H.

Graphical abstract
Similar content being viewed by others
References
Jutzi P, Schubert U (2004) Silicon chemistry: from the atom to extended systems. Angew Chemie 43(23):2990
Denk M, Lennon R, Hayashi R, West R, Belyakov AV, Verne HP, Haaland A, Wagner M, Metzler N (1994) Synthesis and Structure of a Stable Silylene. J Am Chem Soc 116(6):2691–2692
Haaf M, Schmedake TA, West R (2000) Stable Silylenes. Acc Chem Res 33(10):704–714
Hadlington TJ, Abdalla JAB, Tirfoin R, Aldridge S, Jones C (2016) Stabilization of a two-coordinate, acyclic diaminosilylene (ADASi): completion of the series of isolable diaminotetrylenes, :E(NR2)2(E = group 14 element). Chem Commun 52(8):1717–1720
Schmedake TA, Haaf M, Paradise BJ, Millevolte AJ, Powell DR, West R (2001). J Organomet Chem 636(1):17–25
Gehrhus B, Lappert MF (2001). J Organomet Chem 617:209–223
Ayoubi-Chianeh M, Kassaee MZ (2019) Novel silicon super bases at DFT level of theory: effects of fused benzene rings on the basicity of 2,4,6-cycloheptatrienesilylene. Res Chem Intermed 45:4677–4691
Ayoubi-Chianeh M, Kassaee MZ, Ashenagar S, Cummings PT (2019) J Phys Org Chem 32(8):e3956
Ayoubi-Chianeh M, Kassaee MZ 2019 J Phys Org Chem 32(10):e3988
Cote DR, Van Nguyen S, Stamper AK, Armbrust DS, Tobben D, Conti RA, Lee GY (1999). IBM J Res Dev 43(1.2):5–38
Heaven MW, Metha GF, Buntine MA (2001). J Phys Chem A 105(7):1185–1196
Tamao K, Kobayashi M, Matsuo T, Furukawa S, Tsuji H (2012) The first observation of electroluminescence from di(2-naphthyl)disilene, an Si Si double bond-containing π-conjugated compound. Chem Commun 48(7):1030–1032
Schwartz RL, Davico GE, Ramond TM, Lineberger WC (1999). J Phys Chem A 103(41):8213–8221
Luke BT, Pople JA, Krogh-Jespersen M-B, Apeloig Y, Karni M, Chandrasekhar J, Schleyer P v R (1986). J Am Chem Soc 108(2):270–284
Kalcher J, Sax AF (1992) Singlet-triplet splittings and electron affinities of some substituted silylenes. J Mol Struct Theochem 253:287–302
Krogh-Jespersen K (1985) Structural and energetic features of fully substituted silylenes, disilenes, and silylsilylenes (SiX2, X2SiSiX2, and XSiSiX3; X = lithium, methyl, and fluorine). J Am Chem Soc 107(3):537–543
Yoshida M, Tamaoki N (2002) DFT Study on Triplet Ground State Silylenes Revisited: The Quest for the Triplet Silylene Must Go On. Organometallics 21(13):2587–2589
Asay M, Inoue S, Driess M (2011) Aromatic Ylide-Stabilized Carbocyclic Silylene. Angew Chemie Int Ed 50(41):9589–9592
Oláh J, Veszprémi T, De Proft F, Geerlings P (2007). J Phys Chem A 111(42):10815–10823
Rekken BD, Brown TM, Fettinger JC, Tuononen HM, Power PP (2012) Isolation of a Stable, Acyclic, Two-Coordinate Silylene. J Am Chem Soc 134(15):6504–6507
Biswas AK, Lo R, Ganguly B (2013). J Phys Chem. A 117(14):3109–3117
Kosa M, Karni M, Apeloig Y (2004) How to Design Linear Allenic-Type Trisilaallenes and Trigermaallenes. J Am Chem Soc 126(34):10544–10545
Apeloig Y, Pauncz R, Karni M, West R, Steiner W, Chapman D (2003) Why Is Methylene a Ground State Triplet while Silylene Is a Ground State Singlet?†. Organometallics 22(16):3250–3256
Belzner J, Dehnert U, Ihmels H (2001) Reactions of a cyclotrisilane with styrene derivatives and diarylacetylenes—evidence for nucleophilic silylenes. Tetrahedron 57(3):511–517
Dubois I, Herzberg G, Verma RD (1967). J Chem Phys 47(10):4262–4263
Ayoubi-Chianeh M, Kassaee KZ (2020) J Chinese Chem Soc 67(9):1544–1551
Ayoubi-Chianeh M, Kassaee MZ n.d. J Phys Org Chem e4074
Öztürk N, Özdemir T, Alpaslan YB, Gokce H, Alpaslan G (2018). Bilge Int J Sci Technol Res 2(1):56–73
Alvareda E, Denis PA, Iribarne F, Paulino M (2016) Bond dissociation energies and enthalpies of formation of flavonoids: A G4 and M06-2X investigation. Comput Theor Chem 1091:18–23
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su S (1993). J Comput Chem 14(11):1347–1363
Schmidt MW, Gordon MS (2017) J Phys Chem A 121(41):8003–8011
Bryantsev VS, Diallo MS, van Duin ACT, Goddard III WA (2009) Evaluation of B3LYP, X3LYP, and M06-Class Density Functionals for Predicting the Binding Energies of Neutral, Protonated, and Deprotonated Water Clusters. J Chem Theory Comput 5(4):1016–1026
Zhao Y, Truhlar DG (2008). Theor Chem Accounts Theory Comput Model (Theoretica Chim Acta) 120(1):215–241
Walker M, Harvey AJA, Sen A, Dessent CEH (2013). J Phys Chem A 117(47):12590–12600
Domingo LR, Chamorro E, Pérez P (2008). J Org Chem 73(12):4615–4624
Yang W, Parr RG (1985) Hardness, softness, and the fukui function in the electronic theory of metals and catalysis. Proc Natl Acad Sci 82(20):6723–6726
Sheela NR, Muthu S, Sampathkrishnan S (2014) Spectrochim. Acta Part A Mol Biomol Spectrosc 120:237–251
Chandra A, Uchimaru T (2002) The O-H Bond Dissociation Energies of Substituted Phenols and Proton Affinities of Substituted Phenoxide Ions: A DFT Study. Int J Mol Sci 3(4):407–422
Biegler-König F, Schönbohm J (2002). J Comput Chem 23(15):1489–1494
Kumar PSV, Raghavendra V, Subramanian V (2016). J Chem Sci 128(10):1527–1536
Gordon MS (1985) Potential-energy surfaces in singlet and triplet silylene. Chem Phys Lett 114(4):348–352
Bader RFW (2005) The Quantum Mechanical Basis of Conceptual Chemistry. Monatshefte für Chemie/Chemical Mon 136(6):819–854
Bader RFW (1991) A quantum theory of molecular structure and its applications. Chem Rev 91(5):893–928
Yang Q, Yang H, Ding X, Xue W, Sun S (2020) The effect of adsorption and grafting on the acidity of [(HSO3)C3C1im]+[Cl]− on the surface of (SiO2)4O2H4 clusters. J Mol Graph Model 96:107528
Weinhold F, Landis CR, Glendening ED (2016) What is NBO analysis and how is it useful? Int Rev Phys Chem 35(3):399–440
Acknowledgements
We gratefully appreciate Tarbiat Modares University for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 11173 kb)
Rights and permissions
About this article
Cite this article
Ayoubi-Chianeh, M., Kassaee, M.Z. New Hydroxylated Cyclic and Acyclic Silylenes Via DFT. Silicon 13, 3385–3397 (2021). https://doi.org/10.1007/s12633-020-00750-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12633-020-00750-5