Abstract
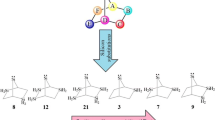
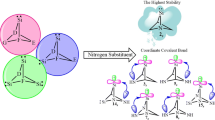
DFT calculations in combination with appropriate isodesmic reactions are employed to assess topological effects of nitrogens on thermodynamic parameters of novel mono-, di-, tri-, tetra-, and pentaaza-7-boratricyclo[1,1,1,01,7,07,3,07,5]hexa-2-silylenes (1–20). Despite the enormous steric strain involved in their cubic structures, all our scrutinized singlet and triplet silylenes (1s-20s vs. 1t-20t, respectively) appear as minima on their energy surfaces, for showing singlet ground states. The highest stability (ΔEs−t) is achieved by 1,3,5-triaza-7-boratricyclo[1,1,1,01,7,07,3,07,5]hexa-2-silylene (11), where all the three nitrogens are bonded to the central boron atom. All of our silylenes show the same trend for their calculated ΔΕs−t and band gap (ΔΕHOMO−LUMO). Isodesmic reactions are employed to compare and contrast nucleophilicity (N), electrophilicity (ω), and heat of hydrogenation (ΔEH) for our 40 silylenes (1s-20s vs. 1t-20t). In fact, we introduce a novel generation of tridimensional silylenes which have the intrinsic potential of expanding the existing boundaries of semiconductors, cumulated multi-dentate ligands, etc..

There are 40 novel borasilylenes with an unprecedented common framework that can accommodate up to five nitrogen heteroatoms. They include singlet (s) and triplet (t) mono-, di, tri-, tetra-, and pentaaza-7-boratricyclo[1,1,1,01,7,07,3,07,5]hexa-2-silylenes. They are compared and contrasted with respect to their geometrical parameters, thermodynamic stabilities, isodesmic reactions, at B3LYP/AUG-cc-pVTZ//B3LYP/6-311 + + G** level of theory. Despite the enormous steric strain involved in their cubic structures, all our scrutinized singlet and triplet silylenes appear as minima on their energy surfaces
Similar content being viewed by others
References
Schoeller WW, Sundermann A, Reiher M (1999) Inorg Chem 38:29–37
Holthausen MC, Koch W, Apeloig Y (1999) J Am Chem Soc 121:2623–2624
Kassaee MZ, Shakib FA, Momeni MR, Ghambarian M, Musavi SM (2010) J Org Chem 75:2539–2545
Hadlington TJ, Driess M, Jones C (2018) Chem Soc Rev 47:4176–4197
Nefedov OM, Egorov MP, Ioffe AI, Menchikov LG, Zuev PS, Minkin VI, Simkin BY, Glukhovstev MN (1992) Pure Appl Chem 64:265–314
Schwartz RL, Davico GE, Ramond TM, Lineberger WC (1999) J Phys Chem A 103:8213–8221
Denk M, Lennon R, Hayashi R, West R, Belyakov AV, Verne HP, Haaland A, Wagner M, Metzler N (1994) J Am Chem Soc 116:2691
Ayoubi-Chianeh M, Kassaee MZ (2019) J Phys Org Chem 32(10):1–15
Heaven MW, Metha GF, Buntine MA (2001) J Phys Chem A 105:1185–1196
Zachariah MR, Tsang W (1995) J Phys Chem 99:5308–5318
Lucas DJ, Curtiss LA, Pople JA (1993) J Chem Phys 99:6697–6703
Boudjouk P, Black E, Kumarathasan R (1991) Organometal 10:2095–2096
Kassaee MZ, Buazar F, Soleimani-Amiri S (2008) J Mol Struct THEOCHEM 866:52–57
Cote DR, Van Nguyen S, Stamper AK, Armbrust DS, Tobben D, Conti RA, Lee GY (1999) IBM J Res Dev 43:5–38
Kassaee MZ, Najafi Z, Shakib FA, Momeni MR (2011) J Organometal Chem 696:2059–2064
Bourissou D, Guerret O, Gabbai FP, Bertrand G (2000) Chem Rev 100:39–92
Mizuhata Y, Sasamori T, Tokitoh N (2009) Chem Rev 109:3479–3511
Nyulaszi L, Belghazi A, Kis-Szetsi S, Veszpremi T, Heinicke J (1994) Theochem 313:73–81
Schoeller WW, Eisner D (2004) Inorg Chem 43:2585–2589
Kirilchuk AA, Rozhenko AB, Leszczynski J (2017) Comp Theor Chem 1103:83–91
Zhou YP, Zh. Mo MPh, Luecke M, Driess (2018) Chem Eur J 24:4780–4784
Zhou YP, Wang Y, Driess M (2017) J Organometal Chem 829:2–10
Brück A, Gallego D, Wang W, Irran E, Driess M, Hartwig JF (2012) Angew Chem Int Ed 51:11478–11482
Schmidt M, Blom B, Szilvasi T, Schomacker R, Driess M (2017) Eur J Inorg Chem 9:1284–1291
Ren H, Zhou YP, Bai Y, Cui C, Driess M (2017) Chem Eur J 23:5663–5667
Tan G, Enthaler S, Inoue Sh, Blom B, Driess M (2015) Angew Chem Int Ed 54:2214–2218
Ayoubi-Chianeh M, Kassaee MZ, Ashenagar S, Cummings PT (2019) J Phys Org Chem 32(8):1–13
Brück A, Gallego D, Wang W, Irran E, Driess M, Hartwig JF (2012) Angew Chem Int Ed 51:11478
Wang D, Choi D, Li J, Yang Z, Nie Z, Kou R, Hu D, Wang C, Saraf LV, Zhang J (2009) ACS Nano 3:907
Fürstner A, Krause H, Lehmann CW (2001) Chem Commun :2372–2373
Yamada T, Mawatari A, Tanabe M, Osakada K, Tanase T (2009) Angew Chemie 121:576
Soleimani Purlak N, Kassaee MZ (2020) J Phys Org Chem 33(6):1–22
Kassaee MZ, Musavi SM, Hamadi H, Ghambarian M, Hosseini SE, J Molecul Struct: THEOCHEM, 2005, 730 33–44
Akbari A, Golzadeh B, Arshadi S, Kassaee MZ (2015) RSC Adv 5:43319–43327
Kassaee MZ, Musavi SM, Ghambarian M (2005) J Mol Struct (Theochem) 731:225–231
Yan Z, Truhlar DG (2008) Theor Chem Account 120:215–241
Becke AD (1988) Phys Rev 38:3098
Becke AD (1993) J Chem Phys 98:5648–5652
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su S, Windus TL, Dupuis M, Montgomery JA (1993) J Comput Chem 14:1347–1363
Adamo C, di Matteo A (1999) Adv Quantum Chem 36:45–75
Zhao Y, Truhlar DG (2008) Acc Chem Res 41(2):157–167
Becke AD (1988) Phys Rev A 38:3098–3100
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Ahangari MG, Mashhadzadeh AH, Fathalian M, Dadrasi A, Rostamiyan Y, Mallahi A (2019) Vacuum 165:26–34
Mashhadzadeh AH, Fereidoon A, Ahangari MG (2017) Superlattices Microstruct 111:23–31
Mashhadzadeh AH, Fereidoon A, Ahangari MG (2017) Mater Chem Phys 201:214–223
Mashhadzadeh AH, Ahangari MG, Salmankhani A, Fataliyan M (2018) Phys E 104:275–285
Ghafari A, Boochani A, Janowitz C, Manzke R (2011) Phys Rev B :84
Afsari M, Boochani A, Hantezadeh M, Elahi SM (2017) Solid State Commun 259:10–15
Lashgari H, Abolhassani MR, Boochani A, Sartipi E, Taghavi-Mendi R, Ghaderi A (2016) Indian J Phys 90:909–916
Kassaee MZ, Ashenagar S (2018) J Mol Model 24:2–11
Domingo LR, Chamorro E, Perez P (2008) J Org Chem 73:4615–4624
Parr RG, Szentpaly L, Liu S (1999) J Am Chem Soc 121:1922–1924
Parr RG, Pearson RG (1983) J Am Chem Soc 105:7512–7516
Kassaee MZ, Najafi Z, Shakib FA, Momeni MR (2011) J Organomet Chem 696:2059–2064
Wang RH, Su MD (2008) J Phys Chem A 112:7689–7698
Martin D, Baceiredo A, Gornitzka H, Schoeller WW, Bertrand G (2005) Angew Chem Int Ed 44:1700–1703
Acknowledgements
The support from Tarbiat Modares University (TMU) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
There are no conflicts to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mohammad Zaman Kassaee is a visiting scholar (sabbatical).
Electronic Supplementary Material
ESM 1
(DOCX 10.7 MB)
Rights and permissions
About this article
Cite this article
Abedini, N., Kassaee, M.Z. & Cummings, P.T. Borasilylenes in Focus: Topological Effects of Nitrogen Atoms by DFT. Silicon 13, 3377–3383 (2021). https://doi.org/10.1007/s12633-020-00745-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12633-020-00745-2