Abstract
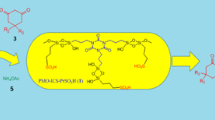
In this study, periodic mesoporous organosilica containing bridges with N-sulfonic acid groups (SA-PMO), was easily prepared in two steps: (i) preparation of periodic mesoporous organosilica containing amine-bridges (AM-PMO) via co-condensation of bis[3-(trimethoxysilyl)propyl] amine and tetraethoxysilane and (ii) functionalization of AM-PMO with sulfonic acid. The formation and morphology of the reagent were confirmed by N2 adsorption-desorption isotherms, pH measurement, X-ray diffraction (XRD), transmission electron microscopy (TEM) and Fourier transform infrared FT-IR analysis. After identification, the catalytic ability of this reagent was investigated in the formylation of amines and alcohols using formic acid. Eco-friendly protocol, excellent yields, short reaction times, reusability of the catalyst and easy and quick isolation of the products are the main advantages of the presented method.
Similar content being viewed by others
References
Fieser LF, Jones JE (1955) N-methylformanilide. Org Synth Coll 3:590–591
Chen BC, Bednarz MS, Zhao R, Sundeen JE, Chen P, Shen Z, Skoumbourdis AP, Barrish AP (2000) A new facile method for the synthesis of 1-arylimidazole-5-carboxylates. Tetrahedron Lett 41:5453–5456
Kobayashi K, Nagato S, Kawakita M, Morikawa O, Konishi H (1995) Synthesis of 1-formyl-1,2-dihydroquinoline derivatives by a Lewis acid-catalyzed cyclization of O-(1-hydroxy-2-alkenyl) phenyl isocyanides. Chem Lett 24:575–576
Lohary BB, Baskaran S, Rao SB, Reddy YB, Rao NI (1999) A short synthesis of oxazolidinone derivatives linezolid and eperezolid: a new class of antibacterials. Tetrahedron Lett 40:4855–4856
Petit RG, Kalnins VM, Liu HMT, Thomas GE, Parent K (1961) Notes- potential cancerocidal agents. iii. Formanilides. J Organomet Chem 26:2563–2566
Kobayashi S, Yasuda M, Hachiya I (1996) Trichlorosilane-dimethylformamide (Cl3SiH-DMF) as an efficient reducing agent. Reduction of aldehydes and imines and reductive amination of aldehydes under mild conditions using hypervalent hydridosilicates. Chem Lett 25:407–408
Kobayashi S, Nishio K (1994) Facile and highly stereoselective synthesis of homoallylic alcohols using organosilicon intermediates. J Organomet Chem 56:6620–6628
Han Y, Cai L (1997) An efficient and convenient synthesis of formamidines. Tetrahedron Lett 38:5423–5426
Hartinez J, Laur J (1982) Active esters of formic acid as useful formylating agents: improvements in the synthesis of formyl-amino acid esters, N-α-Formyl-Met-Leu-Phe-OH, and Formyl-Met-Lys-Pro-Arg, a phagocytosis stimulating peptide. Synthesis 11:979–981
Blicke FF, Lu CJ (1952) Formylation of amines with chloral and reduction of the N-formyl derivatives with lithium aluminum hydride. J Am Chem Soc 74:3933–3934
Jung SH, Ahn JH, Park SK, Choi JK (2002) A practical and convenient procedure for the N-formylation of amines using formic acid. Bull Kor Chem Soc 23:149–150
Das B, Krishnaiah M, Balasubramanyam P, Veeranjaneyulu B, Nandan Kumar D (2008) A remarkably simple N-formylation of anilines using polyethylene glycol. Tetrahedron Lett 49:2225–2227
Hosseini-Sarvari M, Sharghi H (2006) ZnO as a new catalyst for N-formylation of amines under solvent-free conditions. J Organomet Chem 71:6652–6654
Strazzolini P, Giumanini AG, Cauci S (1990) Acetic formic anhydride a review. Tetrahedron 46:1081–1118
Zhou L, Freyschlag CG, Xu B, Friend CM, Madix RJ (2010) Direct selective oxygen-assisted acylation of amines driven by metallic silver surfaces: Dimethylamine with formaldehyde. Chem Commun 46:704–706
Xu B, Zhou L, Madix RJ, Friend CM (2010) Highly selective acylation of dimethylamine mediated by oxygen atoms on metallic gold surfaces. Angew Chem Int Ed 49:394–398
Reddy PG, Kumar GDK, Bhaskaran S (2000) A convenient method for the N-formylation of secondary amines and anilines using ammonium formate. Tetrahedron 41:9149–9151
Hagiwara H, Morohashi K, Sakai H, Suzuki T, Ando M (1998) Acetylation and formylation of alcohols with triphenylphosphine and carbon tetrabromide in ethyl acetate or ethyl formate. Tetrahedron 54:5845–5852
Orita A, Tanahashi C, Kakuda A, Otera J (2000) Highly efficient and versatile acylation of alcohols with Bi (OTf)3 as catalyst. Angew Chem Int Ed 39:2877–2879
Ishihara K, Kubota M, Kurihara H, Yamamoto H (1996) Scandium trifluoromethanesulfonate as an extremely active Lewis acid catalyst in acylation of alcohols with acid anhydrides and mixed anhydrides. J Organomet Chem 61:4560–4567
Shirini F, Marjani K, Taherpour-Nahzomi H, Zolfigol MA (2007) Silica triflate as an efficient reagent for the chemoselective formylation of alcohols. Phosphorus Sulfur Silicon Relat Elem 182:1245–1251
Shirini F, Seddighi M, Mamaghani M (2014) Bronsted acidic ionic liquid supported on rice husk ash (RHA-[pmim]HSO4): a highly efficient and reusable catalyst for the formylation of amines and alcohols. RSC Adv 4:50631–50638
Khazaei A, Rostami A, Mantashlo F (2010) p-Toluenesulfonyl chloride as a new and effective catalyst for acetylation and formylation of hydroxyl compounds under mild conditions. Chin Chem Lett 21:1430–1434
Shirini F, Zolfigol MA, Mallakpour B (2005) Mild and efficient procedure for acetylation and formylation of alcohols in the presence of Mg (HSO4)2. Russ J Org Chem 41:625–626
Shirini F, Zolfigol MA, Safari A (2006) Efficient acetylation and formylation of alcohols in the presence of Zr (HSO4)4. J Chem Res 3:154–156
Ashoka S, Chandrappa GT, Pasha MA (2010) Nano-MgO: an efficient catalyst for the synthesis of formamides from amines and formic acid under MWI. Catal Lett 138:82–87
Bhojegowd MRM, Nizam A, Pasha MA (2010) Amberlite IR-120: a reusable catalyst for N-formylation of amines with formic acid using microwaves. Chin J Catal 31:518–520
Kim JG, Jang DO (2010) Facile and highly efficient N-formylation of amines using a catalytic amount of iodine under solvent-free conditions. Synlett 14:2093–2096
Ma’mani L, Sheykhan M, Heydari A, Faraji M, Yamini Y (2010) Sulfonic acid supported on hydroxyapatite-encapsulated-γ-Fe2O3 nanocrystallites as a magnetically Brønsted acid for N-formylation of amines. Appl Catal A 377:64–69
Inagaki S, Guan S, Fukushima Y, Ohsuna T, Terasaki O (1999) Novel mesoporous materials with a uniform distribution of organic groups and inorganic oxide in their frameworks. J Am Chem Soc 121:9611–9614
Melde BJ, Holland BT, Blanford CF, Stein A (1999) Mesoporous sieves with unified hybrid inorganic/organic frameworks. Chem Mater 11:3302–3308
Asefa T, MacLachlan MJ, Coombs N, Ozin GA (1999) Periodic mesoporous organosilicas with organic groups inside the channel walls. Nature 402:867–871
Yang QH, Liu J, Zhang L, Li C (2009) Functionalized periodic mesoporous organosilicas for catalysis. J Mater Chem 19:1945–1955
Mizoshita N, Tani T, Inagaki S (2011) Syntheses, properties and applications of periodic mesoporous organosilicas prepared from bridged organosilane precursors. Chem Soc Rev 40:789–800
Mizoshita N, Goto Y, Maegawa Y, Tani T, Inagaki S (2010) Tetraphenylpyrene-bridged periodic mesostructured organosilica films with efficient visible-light emission. Chem Mater 22:2548–2554
Goethals F, Meeus B, Verberckmoes A, Van der Voort P, Van Driessche I (2010) Hydrophobic high quality ring PMOs with an extremely high stability. J Mater Chem 20:1709–1716
Haghighat M, Shirini F, Golshekan M (2018) Efficiency of NaHSO4 modified periodic mesoporous organosilica magnetic nanoparticles as a new magnetically separable nanocatalyst in the synthesis of [1,2,4] triazolo quinazolinone/pyrimidine derivatives. J Mol Struct 1171:168–178
Haghighat M, Shirini F, Golshekan M (2019) Synthesis of tetrahydrobenzo [b] pyran and Pyrano [2, 3-d] pyrimidinone derivatives using Fe3O4@ Ph-PMO-NaHSO4 as a new magnetically separable nanocatalyst. J Nanosci Nanotechnol 19:3447–3458
Pourhasan-Kisomi R, Shirini F, Golshekan M (2019) Organic/inorganic Fe3O4@MCM-41@Zr-piperazine: an impressive magnetite nanocatalyst for N-tert-butoxycarbonylation of amines. J Nanosci Nanotechnol 7:3859–3870
Pourhasan-Kisomi R, Shirini F, Golshekan M (2018) Introduction of organic/inorganic Fe3O4@MCM-41@Zr-piperazine magnetite nanocatalyst for the promotion of the synthesis of tetrahydro-4H-chromene and pyrano[2,3-d] pyrimidinone derivatives. Appl Organomet Chem 32:e4371
Esmaeilpour M, Sardarian AR (2014) Dodecylbenzenesulfonic acid as an efficient, chemoselective and reusable catalyst in the acetylation and formylation of alcohols and phenols under solvent-free conditions at room temperature. Iran J Sci Tech Trans A 38:175–186
Ram RN, Meher NK (2002) Selective formylation of alcohols in the presence of phenols with chloral. Tetrahedron 58:2997–3001
Mirkhani V, Tangestaninejad S, Moghadam M, Yadollahi B, Alipanah L (2004) Cerium polyoxometalate as a reusable catalyst for acetylation and formylation of alcohols. Monatsh Chem 135:1257–1263
Brahmachari G, Laskar S (2010) A very simple and highly efficient procedure for N-formylation of primary and secondary amines at room temperature under solvent-free conditions. Tetrahedron Lett 51:2319–2322
Ortega N, Richter C, Glorius F (2013) N-formylation of amines by methanol activation. Org Lett 15:1776–1779
Park HJ, Lee JC (2008) Efficient and solvent-free preparation of formate esters from alcohols under microwave irradiation. Bull Kor Chem Soc 29:856–858
Wang Z, Lu M (2014) Highly efficient N-formylation of amines with ammonium formate catalyzed by nano-Fe3O4 in PEG-400. RSC Adv 4:1234–1240
Habibi D, Nasrollahzadeh M (2013) An ultrasound-promoted green approach for the N-formylation of amines under solvent-and catalyst-free conditions at room temperature. C R Chimie 16:1008–1016
Mihara M, Ishino Y, Minakara S, Komatsu M (2003) Convenient N-formylation of secondary amines: KF-Al2O3-promoted synthesis of formamide derivatives via dichlorocarbene generated from chloroform. Synthesis 15:2317–2320
Niknam K, Saberi D (2009) Silica-bonded N-propyl sulfamic acid as an efficient catalyst for the formylation and acetylation of alcohols and amines under heterogeneous conditions. Tetrahedron Lett 50:5210–5214
Zeynizadeh B, Abdollahi M (2016) The immobilized NaHSO4·H2O on activated charcoal: a highly efficient promoter system for N-formylation of amines with ethyl formate. Curr Chem Lett 5:51–58
Krishnakumar B, Swaminathan M (2011) A convenient method for the N-formylation of amines at room temperature using TiO2-P25 or sulfated titania. J Mol Catal A Chem 334:98–102
Kamer PCJ, Nolte RJM, Drenth W (1988) Screw sense selective polymerization of achiral isocyanides catalyzed by optically active nickel (II) complexes. J Am Chem Soc 110:6818–6825
Acknowledgments
We are thankful to the Research Council of the University of Guilan for the partial support of this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Haghighat, M., Shirini, F. & Golshekan, M. Periodic Mesoporous Organosilica Containing Bridges with N-Sulfonic Acid Groups: a New Catalyst for the Efficient Formylation of Amines and Alcohols. Silicon 12, 2087–2098 (2020). https://doi.org/10.1007/s12633-019-00293-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12633-019-00293-4