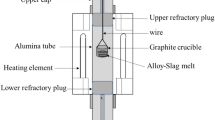
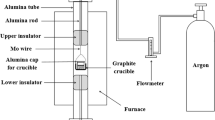
Abstract
In present work, the role of the oxygen potential (PO2) and oxygen ion (O2−) concentration for removing phosphorus (P) during CaO–SiO2–Al2O3–FexO slag refining was studied by on-line measurement of oxygen activity in molten silicon (Si), FactSage calculation, Raman spectroscopy and nuclear magnetic resonance (NMR) spectroscopy. The results show that the addition of FeO from 0 to 9.25 wt% in slag can increase the activity of dissolved oxygen (a[O]) in Si and the mole fraction of O2− in slag. Moreover, the increase of O2− concentration leads to the increase of non-bridge oxygen (NBO). The value of LP (the partition ratio of phosphorous between slag and Si shows a first increase and then decrease trend and reaches a maximum value of 1.95 at 5 ± 0.1 wt% FeO. It is believed that the increase of a[O] and NBO can promote the removal of P as FeO content is less than 5 ± 0.1 wt%. the chain structure unit (Q2) of silicate network as the main intermediate structure to capture PO43− from the charge compensation of P2O5 by O2− to form the sheet structure unit Q3(Si and P). When FeO content is increased to more than 5 ± 0.1 wt%, LP value gradually decreases although the values of NBO and a[O] are increasing. NBO plays a leading role in this process, it can be speculated that more NBO can depolymerize the Q3 (Si and P) to destroy the stability of P in silicate network. As a result, a mount of PO43− is present at the interface to prevent the oxidation of phosphorous, which leads to the decrease of LP value.
Similar content being viewed by others
References
Luque A, Hegedus S (2003) Handbook of photovoltaic science and engineering. Wiley Ltd, New York Chapter 5
Sarti D, Einhaus R (2002) Silicon feedstock for the multi-crystalline photovoltaic industry. Sol Energ Mat Sol C 72:27–40
Sergiienko SA, Pogorelov BV, Daniliuk VB (2014) Silicon and silicon carbide powders recycling technology from wire-saw cutting waste in slicing process of silicon ingots. Sep Purif Technol 133:16–23
Hachichi K, Lami A, Zemmouri H, Cuellar P, Soni R, Ait-Amar H, Drouiche N (2018). Silicon 10:1579–1589
Pushpavanam M, Manikandan H, Ramanathan K (2007) Preparation and characterization of nickel–cobalt-diamond electro-composites by sediment co-deposition. Surf Coat Technol 201:6372–6379
Liu S, Huang K, Zhu H (2016) Removal of Fe, B and P impurities by enhanced separation technique from silicon-rich powder of the multi-wire sawing slurry. Chem Eng J 299:276–281
Lu T, Tan Y, Li J, Deng D (2018) Recycling of silicon powder waste cut by a diamond-wire saw through laser-assisted vacuum smelting. J Clean Prod 203:574–584
Tomono Z, Miyamoto S, Ogawa T, Furuya H, Okamura Y, Yoshimoto M, Komatsu R, Nakayama M (2013) Recycling of kerf loss silicon derived from diamond-wire saw cutting process by chemical approach. Sep Purif Technol 120:304–309
Lin Y, Wang T, Lan C, Tai CY (2010) Recovery of silicon powder from kerf loss slurry by centrifugation. Powder Technol 200:216–223
Wang TY, Lin YC, Tai CY, Sivakumar R, Rai DK, Lan CW (2008) A novel approach for recycling of kerf loss silicon from cutting slurry waste for solar cell applications. J Cryst Growth 310:3403–3406
Jia G, Plentz J, Gawlik A, Azar AS, Stokkan G, Syvertsen M, Carvalho PA, Dellith J, Dellith A, Andrä G, Ulyashin A (2016). Int J Photoenergy 19:7582–7589
Suzuki K, Kumagal T, Sano N (1992) Removal of boron from metallurgical-grade silicon by applying the plasma treatment. ISIJ Int 32:630–634
Ikeda T, Maeda M (1992) Purification of metallurgical silicon for solar-grade silicon by electron beam button melting. ISIJ Int 32:635–642
Momokawa H, Sano N (1982) The effect of oxygen potential on phosphorus in the CaO−Al2O3 system. Metall Mater Trans B Process Metall Mater Process Sci 13:643–644
Tanahashi M, Fujisawa T (2013). Metall Mater Trans B Process Metall Mater Process Sci 45:629–642
Jung EJ, Moon BM, Min DJ (2011) Quantitative evaluation for effective removal of phosphorus for SoG-Si. Sol Energy Mater Sol Cells 95:1779–1784
Johnston M, Barati M (2010) Distribution of impurity elements in slag–silicon equilibria for oxidative refining of metallurgical silicon for solar cell applications. Sol Energ Mater Sol Cells 94:2085–2090
Teixeira LAV, Morita K (2009) Removal of boron from molten silicon using CaO–SiO2 based slags. ISIJ Int 49:783–787
Wu JJ, Wang FM, Ma WH (2016) Thermodynamics and kinetics of boron removal from metallurgical grade silicon by addition of high basic potassium carbonate to calcium silicate slag. Metall Mater Trans B Process Metall Mater Process Sci 47:1796–1803
Krystad E, Tang K, Tranell G (2012). JOM. 64:968–972
Li FS, Li XP, Yang SF (2017) Distribution ratios of phosphorus between CaO-FeO-SiO2-Al2O3/Na2O/TiO2 slags and carbon-saturated iron. Metall Mater Trans B Process Metall Mater Process Sci 48:2367–2378
Nagabayashi R, Hino M, Ban-Ya S (1989) Mathematical expession of phosphorus distribution in steelmaking process by quadratic formalism. ISIJ Int 29:140–147
Danaei A, Yang YD, Barati MC, Ravindran R (2013). Mater Sci Technol 27 CD only
Pak JJ, Fruehan RJ (1986) Soda slag system for hot metal dephosphorization. Metall Mater Trans B Process Metall Mater Process Sci 17:797–804
Yang XM, Shi CB, Zhang M (2011) A thermodynamic model of phosphate capacity for CaO-SiO2-MgO-FeO-Fe2O3-MnO-Al2O3-P2O5 slags equilibrated with molten steel during a top–bottom combined blown converter steelmaking process based on the ion and molecule coexistence theory. Metall Mater Trans B Process Metall Mater Process Sci 42:951–977
Morales AT, Fruehan RJ (1997) Thermodynamics of MnO, FeO, and phosphorus in steelmaking slags with high MnO contents. Metall Mater Trans B Process Metall Mater Process Sci 28:1111–1118
Qian GY, Wang Z, Gong XZ (2017) The importance of slag structure to boron removal from silicon during the refining process: insights from raman and nuclear magnetic resonance spectroscopy study. Metall Mater Trans B Process Metall Mater Process Sci 48:3239–3250
Teixeira LAV, Tokuda Y, Yoko T, Morita K (2009) Behavior and state of boron in CaO–SiO2 slags during refining of solar grade silicon. ISIJ Int 49:777–782
Tsunawaki Y, Iwamoto N, Hattori T, Mitsuishi A (1981) Analysis of CaO · SiO2 and CaO · SiO2 · CaF2 glasses by Raman spectroscopy. J Non-Cryst Solids 44:369–378
Sun YQ, Zhang ZT (2015). Metall Mater Trans B Process Metall Mater Process Sci 46B:1549–1554
Seifert FA, Mysen BO, Virgo D (1982). Am Mineral 67:696–717
Tan J, Zhao SR, Wang WF (2004) The effect of cooling rate on the structure of sodium silicate glass. Mater Sci Eng B 106:295–299
Wang ZJ, Shu Q (2015) Effect of P2O5 and FetO on the viscosity and slag structure in steelmaking slags. Metall Mater Trans B Process Metall Mater Process Sci 46:758–765
McIntyre NS, Zetaruk DG (1977) X-ray photoelectron spectroscopic studies of iron oxides. Anal Chem 49:1521–1529
Hawn DD, DeKoven BM (1987) Deconvolution as a correction for photoelectron inelastic energy losses in the core level XPS spectra of iron oxides. Surf Interface Anal 10:63–74
Mills P, Sullivan JL (1983) A study of the core level electrons in iron and its three oxides by means of X-ray photoelectron spectroscopy. J Phys D Appl Phys 16:723–732
Langevoort JC, Sutherland I, Hanekamp LJ, Gellings PJ (1987) On the oxide formation on stainless steels AISI 304 and incoloy 800H investigated with XPS. Appl Surf Sci 28:167–179
Tan BJ, Klabunde KJ, Sherwood PA (1990) X-ray photoelectron spectroscopy studies of solvated metal atom dispersed catalysts. Monometallic iron and bimetallic iron-cobalt particles on alumina. Chem Mater 2:186–191
Lockyer MWG, Holland D, Dupree R (1995) NMR investigation of the structure of some bioactive and related glasses. J Non-Cryst Solids 188:207–219
Fayon F, Massiot D, Suzuya K, Price DL (2001) 31P NMR study of magnesium phosphate glasses. J Non-Cryst Solids 283:88–94
Tilocca A, Cormack AN, de Leeuw NH (2007) The Structure of Bioactive Silicate Glasses: New Insight from Molecular Dynamics Simulations. Chem Mater 19:95–103
Mysen BO, Ryerson FJ, Virgo D (l981). Am Mineral 66:106–117
Kline J, Tangstad M, Tranell G (2015). Metall Mater Trans B Process Metall Mater Process Sci 46B:62–73
Tillmanns E, Gebert W, Baur WH (1973). J Solid State Chem 7:69–84
Poojary DM, Borade RB, Campbeii III FL, Clearfield A (1994). J Solid State Chem 112:106–112
Elgayar I, Aliev AE, Boccaccini AR, Hill RG (2005) Structural analysis of bioactive glasses. J Non-Cryst Solids 351:173–183
Dupree R, Holland D, Mortuza MG, Collins JA, Lockyer MWG (1989) Magic angle spinning NMR of alkali phospho-alumino-silicate glasses. J Non-Cryst Solids 112:111–117
Tilocca A, Cormack AN (2007) Structural effects of phosphorus inclusion in bioactive silicate glasses. J Phys Chem B 111:14256–14264
Martynov PV, Chernov ME, Levskit VA (2005). Atom Energy 98:343–346
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (Nos. 51604256 and U1702251), National Key R&D Program of China (2018YFC1901801) and Beijing Natural Science Foundation (2192055).
Author information
Authors and Affiliations
Author notes
Guoyu Qian and Nan Zhang Thurmond are co-first authors.
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, N., Qian, G., Wang, Z. et al. Role of Oxygen Potential and Oxygen Ions on Phosphorus Removal from Silicon via Addition of FeO into Slag. Silicon 12, 1145–1156 (2020). https://doi.org/10.1007/s12633-019-00201-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12633-019-00201-w