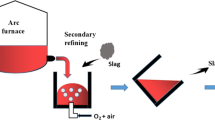
Abstract
A new electro-thermal metallurgy method to prepare high purity silicon (ASTM A 922) was developed in this paper. CaO-SiO2 were added into raw materials as slagging agents during the smelting process in order to improve the purity of the silicon products. This method combined the smelting process with the slag refining process into one step. It not only simplified the technological process, but also decreased the production cost of SoG-Si significantly. In this study, the distribution of impurity elements in different areas of the crucible was studied and the mechanism of impurity elements removed by the slag phase was also investigated. Phosphorus (P) in liquid Si could diffuse into the slag/Si interface and react with SiO2 which was derived from the melted slag phase. Then the reaction product P2O5 would diffuse into the melted slag phase from slag/Si interface and react with CaO to form the stable compound 3CaO ·P2O5. Compared to the result without adding slagging agents, the concentration of elements Fe, Al, P and S was reduced by 90.71%, 63.90%, 44.52% and 56.93% with this new method. The purity of silicon product can reach up to 99.6%. The results indicate that adding slagging agent into the raw materials during the smelting process is beneficial to remove impurity elements and improve the purity of the silicon product.
Similar content being viewed by others
References
Clarson SJ (2016) Modern aspects of energy and materials. Silicon 9(1):1–5
Lynch D (2009) Winning the global race for solar silicon. JOM 61(11):41–48
Iacono ST, Clarson SJ (2014) Silicon and energy. Silicon 6(4):211–213
Bye G, Bruno C (2014) Solar grade silicon: Technology status and industrial trends. Solar Energy Mater Solar Cells 130:634–646
Bu X (2008) Market status and development trend of silicone industry. Chem Ind 26(7):57–58
Xu H, Shen H, Liang Z (2008) Production and development status of solar grade poly silicon. Mater Rev 22(9):86–90
Ramos A, Filtvedt WO, Lindholm D et al (2015) Deposition reactors for solar grade silicon: A comparative thermal analysis of a Siemens reactor and a fluidized bed reactor. J Cryst Growth 431:1–9
Reznichenko M (2016) Evolution of Requirements for Solar Grade Silicon. Proced Eng 139:41–46
Yadav S, Chattopadhyay K, Singh CV (2017) Solar grade silicon production: A review of kinetic, thermodynamic and fluid dynamics based continuum scale modeling. Renew Sust Energ Rev 78:1288–1314
Johnston MD, Khajavi LT, Li M et al (2012) High-temperature refining of metallurgical-grade silicon: a review. JOM 64(8):935–945
Fidelis C (2017) From metallurgical-grade to solar-grade silicon: an overview. Silicon 1:1–10
Feng LI, Xing PF, Da-Gang LI et al (2013) Removal of phosphorus from metallurgical grade silicon by Ar-H2O gas mixtures. Trans Nonferrous Metals Soc China 23(11):3470–3475
Li Y, Wu J, Ma W et al (2015) Boron removal from metallurgical grade silicon using a refining technique of calcium silicate molten slag containing potassium carbonate. Silicon 7(3):1–6
Ding C, Ma W, Wei K et al (2013) Latest progress in purification of metallurgical grade silicon by slag oxidation refining. Chin J Vac Sci Technol 33(2):185–191
Lu T, Tan Y, Shi S et al (2017) Continuous electron beam melting technology of silicon powder by prefabricating a molten silicon pool. Vacuum 143:336–343
Ren S, Li P, Jiang D et al (2015) Removal of Cu, Mn and Na in multicrystalline silicon by directional solidification under low vacuum condition. Vacuum 115:108–112
Liu T, Dong Z, Zhao Y et al (2012) Purification of metallurgical silicon through directional solidification in a large cold crucible. J Cryst Growth 355(1):145–150
Li P, Ren S, Jiang D et al (2017) Distributions of substitutional and interstitial impurities in silicon ingot with different grain morphologies. Mater Sci Semicond Process 67:1–7
Sarangi M (2009) Effect of an Iron Catalyst and Process Parameters on Si-Based Ceramic Materials Synthesized From Rice Husks. Silicon 1(2):103–109
Xu W, Lo TY, Memon SA (2012) Microstructure and reactivity of rich husk ash. Constr Build Mater 29(4):541–547
Bakar RA, Yahya R, Gan SN (2016) Production of high purity amorphous silica from rice husk. Proced Chem 19:189–195
Ahmed MZY, Ewais ME, Zaki IZ (2008) Production of porous silica by the combustion of rice husk ash for tundish lining. Int J Miner Metall Mater 15(3):307–313
Marchal JC, Mcdonnell P, Sun K et al (2015) A low cost, low energy route to solar grade silicon from rice hull ash (RHA), a sustainable source. Green Chem 17(7):3931–3940
Practical technology of industrial silicon writing group (2005) Practical technology of industrial silicon. Chemical Industry Press, Beijing, pp 67–121
Filsinger DH, Bourrie DB (1990) ChemInform abstract: silica to silicon: key carbothermic reactions and kinetics. ChemInform 73(36):1726–1732
Pan Z, Dai Z, Xu L et al (2001) Temperature-controlled growth of silicon-based nanostructures by thermal evaporation of SiO powders. J Phys Chem B 105(13):2507–2514
Zhu M (2005) Modern metallurgical technology: ferrous metallurgy volume. Metallurgical Industry Press, Beijing, pp 20–196
Martello ED, Tranell G, Ostrovski O et al (2013) Trace elements in the Si furnace. part i: behavior of impurities in quartz during reduction. Metall Mater Trans B 44(2):233–243
Whittaker EJW, Muntus R (1970) Ionic radii for use in geochemistry. Geochim Cosmochim Acta 34(9):945–956
Huang L, Lai H, Gan C et al (2016) Separation of boron and phosphorus from Cu-alloyed metallurgical grade silicon by CaO–SiO2–CaCl2 slag treatment. Sep Purif Technol 170:408– 416
Johnston MD, Barati M (2010) Distribution of impurity elements in slag–silicon equilibria for oxidative refining of metallurgical silicon for solar cell applications. Solar Energy Mater Solar Cells 94(12):2085–2090
Acknowledgments
This work was financially supported by the State Key Program of the National Natural Science Foundation of China (Grant No.51334004), the National Natural Science Foundation of China (Grant No.51074043).
Author information
Authors and Affiliations
Corresponding author
Additional information
Study of the preparation of high purity silicon by a new electro-thermal metallurgy method.
Highlights A new process with addition of CaO-SiO2 slagging agents to prepare high purity silicon was investigated.
Preparation of high purity silicon from silica sand and CRH using an electric arc furnace was studied and reaction areas of the crucible with five regions were analyzed.
The mechanism of impurity elements removed by slag was discussed through the experimental results and thermodynamic analysis.
Rights and permissions
About this article
Cite this article
Ye, K., Wang, J., Xing, P. et al. Study of the Preparation of High Purity Silicon by a New Electro-thermal Metallurgy Method. Silicon 11, 1175–1184 (2019). https://doi.org/10.1007/s12633-017-9679-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12633-017-9679-x