Abstract
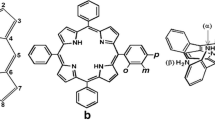
Mesoporous silica is a very suitable carrier for preparation of sensors for detection of explosive compounds with high selectivity and rapid responsiveness by incorporating chemical sensing units into silica films. Three types of porphyrin-doped silica with mesoporous and amorphous structures were prepared. 5,10,15,20-tetrakis(4-carboxyphenyl)porphyrin was synthesized in two steps starting from p-methoxycarbonylbenzaldehyde. 5,10,15,20-tetrakis(4-(methoxycarbonyl) phenyl) porphyrin (1a) was hydrolyzed with trifluoroacetic acid (TFA) to produce 5,10,15,20-tetrakis(4-carboxyphenyl) porphyrin (1b) in 100 % yield. Different kinds of silica such as Hexagonal SBA-15 (SBA-15(Hex)), Spherical SBA-15 (SBA-15(Sp)) and commercial SiO2(SiO2(Ald)) were functionalized using 3-aminopropyltriethoxysilane (3-APS). Porphyrin with carboxylic substituent was reacted with N-hydroxysuccinimide (NHS) and N, N'-dicyclohexylcarbodiimide (DCC) to produce 5-[4-(succinimidyloxycarbonyl)phenyl]-10,15,20-tri(4-carboxyphenyl)porphyrin (H 2 SPTCPP) and grafted to the surface of modified silica to obtain porphyrin-doped silica materials. Results suggest that the synthetic route achieves a high content of porphyrin incorporated to the support. The percentage of porphyrin incorporated to the support, which is higher in the case of SBA-15(Sp) with higher surface area and containing a larger number of silanol groups available for grafting, is directly related to the catalytic activity. Time-dependent fluorescence quenching of porphyrin-doped silica films was studied for three different mesostructures and it showed a rapid quenching upon exposure to a trace amount of TNT vapor due to the high surface area and highly ordered structure of silica.
Similar content being viewed by others
References
Johanson U, Marandi M, Sammelselg V, Tamm J (2005) J Electroanal Chem 575:267–273
Ghiaci M, Molaie F, Sedaghat ME, Dorostkar N (2010) Catal Comm 11:694–699
Zhang L, Sun T, Ying JY (1999) Chem Commun 1103–1104. doi:10.1039/A900629J
Gao B, Wang R, Zhang Y (2009) J App Polym Sci 112:2764–2772
Anzenbacher P, Král V, Jursíková K, Günterová J, Kasal A (1997) J Mol Catal A 118:63–68
Yu XQ, Huang JS, Yu WY, Che CM (2000) J Am Chem Soc 122:5337–5342
Sacco HC, Iamamoto Y, Lindsay Smith JR (2001) J Chem Soc Perkin Trans 2:181
Neys PEF, Vankelecom IFJ, Parton RF, Cenlemans E, Dehaen W, L’abbé G, Jacobs PA (1998) J Mol Catal A 134:209–214
Sacco HC, Ciuffi KJ, Biazzotto JC, Zuccki MR, Leite CAP, Nascimento OR, Serra OA, Iamamoto Y (2000) J Non-Cryst Solids 273:150–158
Fa HB, Zhao L, Wang XQ, Yu JH, Huang YB, Yang M, Wang DJ (2006) Eur J Inorg Chem 21:4355–4361
Moghadam M, Tangestaninejad S, Habibi MH, Mirkhani V (2004) J Mol Catal A 274:217–223
Mukherjee M, Ray AR (2007) J Mol Catal A 266:207–214
Naik R, Joshi P, Umbarkar S, Deshpande RK (2005) Catal Commun 6:125–129
Fuchter MJ, Hoffman BM, Barrett AGM (2006) J Org Chem 71:724–729
Yamada T, Zhou H, Uchida H, Honma I, Katsube T (2004) J Phys Chem B 108:13341–13346
Fan H, Lu Y, Stump A, Reed ST, Baer T, Schunk R, Perez-Luna V, Lopez GP, Brinker J (2000) Nature 405:56–60
Hüsing N, Schubert U (1998) Angew Chem Int Ed 37:22–45
Lee SK, Okura I (1997) Analyst 22:81–84
Tao S, Li G (2007) Colloid Polym Sci 285:721
Liu Y, Mills RC, Boncella JM, Schanze KS (2001) Langmuir 17:7452
Lan EH, Dunn B, Zink JI (2000) Chem Mater 12:1874–1878
Yang JS, Swager TM (1998) J Am Chem Soc 120:11864–11873
Mcquade DT, Pullen AE, Swager TM (2000) Chem Rev 100:2537–2574
Sohn H, Sailor MJ, Magda D, Trogler WC (2003) J Am Chem Soc 125:3821–3830
Rouhi AM (2005) Chem Eng News 83:8
Chang CP, Chao CY, Huang JH, Hsu CS, Lin MS, Hsieh BR, Su AC (2004) Synth Met 144:297–301
Yang JS, Swager TM (1998) J Am Chem Soc 120:5321–5322
Adler AD, Longo FR, Finarelli JD, Goldmacher J, Assour J, Korsakoff L (1967) J Org Chem 32:476
Mohamadnia Z, Ahmadi E, Nekoomanesh M, Ramazani A, Salehi Mobarakeh H (2010) Polym Int 59:945–953
Zhao D, Huo Q, Feng J, Chmelka BF, Stucky GD (1998) J Am Chem Soc 120:6024–6036
Ma Y, Qi L, Ma J, Wu Y, Liu O, Cheng H (2003) Colloids Surf A Physicochem Eng Asp 229:1–8
Zheng S, Gao L, Guo J (2000) J Solid-State Chem 152:447–452
Tao S, Shi Z, Li G, Li P (2006) Chem Phys Chem 7:1902–1905
Rose A, Zhu Z, Madigan CF, Swager TM, Bulovic V (2005) Nature 434:876–879
Toal SJ, Trogler WC (2006) J Mater Chem 16:2871–2873
Tao S, Li G, Zhu H (2006) J Mater Chem 16:4521–4528
Handbook of physical properties of organic chemicals In: Howard PH, Meylan WM (eds). CRC Press, Lewis Publishers (1997)
Luts T, Suprum W, Hofmann D, Klepel O, Papp H (2007) J Mol Catal A: Chem 261:16–23
Qu R, Wang M, Sun C, Zhang Y, Ji C, Chen H (2008) Appl Surf Sci 255:3361–3370
Fagadar-Cosma E, Enache C, Dascalu D, Fagadar-Cosma G, Gavrila R (2008) Optoelectron Adv Mater Rapid Commun 7:437–441
Innocenzi P, Abdirashid MO, Guglielmi M (1994) J Sol–Gel Sci Technol 3:47–55
Al-Oweini R, El-Rassy H (2009). J Mol Struct 919:140–145
Hajji P, David L, Gerard JF, Pascault JP, Vigier GJ (1999) Polym Sci Ser B 37:3172–3187
Cherian S, Wamser CC (2000) J Phys Chem B 104:3624–3629
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Ahmadi, E., Ramazani, A., Hamdi, Z. et al. 5,10,15,20-tetrakis(4-carboxyphenyl)porphyrin Covalently Bound to Nano-silica Surface: Preparation, Characterization and Chemosensor Application to Detect TNT. Silicon 7, 323–332 (2015). https://doi.org/10.1007/s12633-015-9304-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12633-015-9304-9