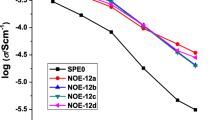
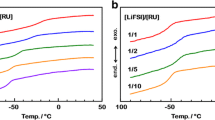
Abstract
Solvent-free polymer electrolytes are critical for improving the performance of electrochemical devices. With the aim of developing a new silicon-based polymer electrolyte that does not contain poly(ethylene oxide) or ionic-liquid moieties, we present the synthesis, spectroscopic, thermogravimetric, and electrochemical characterization of a polymer combining flexible polysiloxanes with polar silatrane moieties at their chain ends or at their pendant chain ends prepared via hydrosilylation. The polymers obtained readily dissolve lithium bis (trifluoromethylsulfonyl)amide (LiTFSA), whereas lithium trifluoromethylsulfonate (LiOTf) and lithium bis(oxalato)borate (LiBOB) exhibit lower solubility. The polysiloxane with silatrane chain ends show an ionic conductivity of about 10 −6 S cm −1 at ambient temperature, a wide electrochemical stability of 5.4 V, a high lithium-ion transference number of 0.70, and good long-time thermal stability up to 150 °C. The pendant-type polymers show lower ionic conductivity because of their high glass transition temperature. Despite their low conductivity, the solvent-free polymer/LiTFSA complexes might find application as binder materials.
Similar content being viewed by others
References
Kamaya N, Homma K, Yamakawa Y, Hirayama M, Kanno R, Yonemura M, Kamiyama T, Kato Y, Hama S, Kawamoto K, Mitsui A (2011) A lithium superionic conductor. Nat Mater 10:682–686
Tatsumisago M, Hayashi A (2012) Superionic glasses and glass-ceramics in the Li 2S-P 2 S 5 system for all-solid-state lithium secondary batteries. Solid State Ionics 225:342–345
Yoshida K, Nakamura M, Kazue Y, Tachikawa N, Tsuzuki S, Seki S, Dokko K, Watanabe M (2011) Oxidative-stability enhancement and charge transport mechanism in glyme-lithium salt equimolar complexes. J Am Chem Soc 133:13121–13129
Yoshida K, Tsuchiya M, Tachikawa N, Dokko K, Watanabe M (2012) Correlation between battery performance and lithium ion diffusion in glyme-lithium bis(trifluoromethanesulfonyl)amide equimolar complexes. J Electrochem Soc 159:A1005–A1012
Rossi RAA, West R (2009) Silicon-containing liquid polymer electrolytes for application in lithium ion batteries. Polym Int 58:267–272
Fish D, Khan IM, Smid J (1986) Conductivity of silid complexes of lithium perchlorate with poly{[ ω-methoxyhexa (oxyethylene)ethoxy]methylsiloxane}. Makromol Chem Rapid Commun 7:115–120
Spindler R, Shriver DF (1988) Investigations of a siloxane-based polymer electrolyte employing 13C, 29Si, 7Li and 23Na solid state NMR spectroscopy. J Am Chem Soc 110:3036–3043
Zhou G-B, Khan IM, Smid J (1993) Solvent-free cation-conducting polysiloxane electrolytes with pendant oligo(oxyethylene) and sulfonate groups. Macromolecules 26:2202–2208
Siska DP, Shriver DF (2001) Li + conductivity of polysiloxane-trifluoromethylsulfonamide polyelectrolytes. Chem Mater 13:4698–4700
Amine K, Wang Q, Vissers DR, Zhang Z, Rossi NAA, West R (2006) Novel silane compounds as electrolyte solvents for Li-ion batteries. Electrochem Commun 8:429–433
Walkowiak M, Schroeder G, Gierczyk B, Waszak D, Osińska M (2007) New lithium ion conducting polymer electrolytes based on polysiloxane grafted with Si-tripodand centers. Electrochem Commun 9:1558–1562
Doyle M, Fuller TF, Newman J (1994) The importance of the lithium ion transference number in lithium/polymer cells. Electrochim Acta 39:2073–2081
Shodai T, Owens BB, Ohtsuka H, Yamaki J-I. (1994) Thermal stability of the polymer electrolyte (PEO) 8LiCF 3 SO 3. J Electrochem Soc 141:2978–2981
Zhu Z, Einset AG, Yang C-Y, Chen W-X, Wnek GE (1994) Synthesis of polysiloxanes bearing cyclic carbonate side chains. Dielectric properties and ionic conductivities of lithium triflate complexes. Macromolecules 27:4076–4079
Lee IJ, Song GS, Lee WS, Suh DH (2003) A new class of solid polymer electrolyte: synthesis and ionic conductivity of novel polysiloxane containing allyl cyanide groups. J Power Sources 114:320–329
Zhang Z, Lyons LJ, West R, Amine K, West R (2005) Synthesis and ionic conductivity of mixed substituted polysiloxanes with oligoethyleneoxy and cyclic carbonated substituents. Silicon Chemistry 3:259–266
Rossi NAA, Wang Q, Amine K, West R (2010) Silicon-containing cabonates- Synthesis, characterization, and additive effects for silicon-based polymer electrolytes. Silicon 2:201–208
Liang S, Choi UH, Liu W, Runt J, Colby RH (2012) Synthesis and lithium ion conduction of polysiloxane single-ion conductors containing novel weak-binding borates. Chem Mater 24:2316–2323
Mizumo T, Fujita R, Ohshita J (2011) Lithium ion conduction in silatrane matrices. Chem Lett 40:798–800
Frye CL, Vogel GE, Hall JA (1961) Triptych-siloxazolidines: pentacoordinate bridgehead silanes resulting from transannular interaction of nitrogen and silicon. J Am Chem Soc 83:996–997
Frye CL, Vincent GA, Finzel WA (1971) Pentacoordinate silicon compounds. V. Novel silatrane chemistry. J Am Chem Soc 93: 6805–6811
Voronkov MG, Dyakov VM, Kirpichenko SV (1982) Silatranes. J Organomet Chem 233:1–147
Puri JK, Singh R, Chahal VK (2011) Silatranes: a review on their synthesis, structure, reactivity and applications. Chem Soc Rev 40:1791–1840
Laine RM, Treadwell DR, Mueller BL, Bickmore CR, Waldner KF, Hinklin TR (1996) Processable aluminosilicate alkoxide precursors from metal oxides and hydroxides. The oxide one-pot synthesis process. Mater Chem Commun 6:1441–1443
Cheng H, Laine RM (1999) Simple, low-cost synthetic route to potentially polymerizable silatranes. New J Chem 23:1181–1186
Armand MB, Bruce PG, Forsyth M, Scrosati B, Wieczorek W (2011) Polymer electrolytes In: Bruce DW, O’Hare D, Walton RI (eds) Energy materials, Wiley, Chichester pp 1–31
Mizumo T, Kajihara T, Yamada T, Ohshita J (2013) Preparation and utilization of poly(methacryloylsilatrane) as a saltdissociation enhancer in PEO-based polymer electrolytes. Polym Adv Technol 24:705–714
Evans J, Vincent CA, Bruce PG (1987) Electrochemical measurement of transference numbers in polymer electrolytes. Polymer 28:2324–2328
Egorov YP, Voronkov MG, Lutsenko TB, Zelchan GI (1966) Infrared absorption spectra of silatranes. Khim Geterotsikl Soedin 2:24–33
Cai D, Neyer A, Kuckuk R, Heise HM (2010) Raman, mid-infrared, near-infrared and ultraviolet-visible spectroscopy of PDMS silicone rubber for characterization of polymer optical waveguide materials. J Mol Struct 976:274–281
Smith BC (1998) Infrared spectral interpretation. CRC Press, Boca Raton
Imura K (2012) Non-aqueous electrolytes containing fluorine. Molten Salts 55:99–102
Barthel J, Gores HJ, Neueder R, Schmid A (1999) Electrolyte solutions for technology-new aspects and approaches. Pure Appl Chem 71:1705–1715
Markusson H, Johansson P, Jacobsson P (2005) Electrochemical stability and lithium ion-anion interactions of orthoborate anions (BOB, MOB, BMB), and presentation of novel anion: tris-oxalato-phosphate. Electrochem Solid-State Lett 8:A215–A218
Johansson P, Jacobsson P (2001) New lithium salts on the computer: fiction or fact?. Electrochim Acta 46:1545–1552
Torell LM, Jacobsson P, Sidebottom D, Petersen G (1992) The importance of ion-polymer crosslinks in polymer electrolytes. Solid State Ionics 53–56:1037–1043
Sylla S, Sanchez JY, Armand M (1992) Electrochemical study of linear and crosslinked POE-based polymer electrolytes. Electrochim Acta 37:1699–1701
Ohno H (2008) Physical properties of ionic liquids for electrochemical applications In: Endres F, Abbott AP, MacFarlane DR (eds) Electrodeposition from ionic liquids, Wiley, Weinheim, pp 47–82
Takekawa T (2010) Approach and task of ionic liquid electrolyte for fuel cell under high temperature and non-humidification. Molten Salts 53:110–114
West R, Whatley LS, Lake KJ (1961) Hydrogen bonding studies. V. The relative basicities of ethers, alkoxysilanes and siloxanes and the nature of the silicon-oxygen bond. J Am Chem Soc 83:761–764
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mizumo, T., Nakashima, M. & Ohshita, J. Oligosiloxanes with Silatrane Moieties for Use in Lithium-ion Conductive Matrices. Silicon 9, 85–96 (2017). https://doi.org/10.1007/s12633-014-9187-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12633-014-9187-1