Abstract
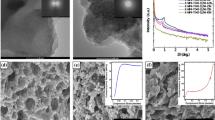
We report the synthesis of highly porous hybrid silica-polyacrylamide aerogels where the inorganic network was obtained through the hydrolysis and poly-condensation of tetramethoxysilane via a two-step sol–gel process while the polyacrylamide polymer was made by photo-copolymerization of two organic monomers, the acrylamide and the bis-acrylamide. These aerogels were obtained after a carbon dioxide supercritical drying while the corresponding xerogels were dried by simple evaporation. These materials, as well as pure silica and polyacrylamide aerogels and xerogels, were characterized by FTIR spectroscopy, solid-state 29Si and 13C NMR spectroscopy, Thermogravimetric Analysis, a nitrogen adsorption-desorption technique, and Scanning Electron Microscopy. The FTIR and NMR spectra and the TGA/DTA analyses confirm the coexistence of highly branched silica and polyacrylamide networks reflecting the hybrid nature of the materials obtained. Nitrogen adsorption measurements reveal high specific surface areas and pore size distributions disclosing the mesoporous character of these hybrid materials. Hybrid silica-polyacrylamide aerogels having a specific surface area equal to 572 m2/g and a pore volume 1.92 cm3/g were successfully prepared for the first time in this study. The high porosity of these aerogels is due to a better resistance of the silica network to capillary forces during the supercritical drying when silica coexists with a polyacrylamide network.
Similar content being viewed by others
References
Loy DA, Shea KJ (1995) Bridged polysilsesquioxanes. Highly porous hybrid organic-inorganic materials. Chem Rev 95(5):1431–1442
Jeong A-Y, Koo S-M, Kim D-P (2000) Characterization of hydrophobic SiO2 powders prepared by surface modification on wet gel. J Sol-Gel Sci Technol 19(1):483–487
Sun J, Akdogan EK, Klein LC, Safari A (2007) Characterization and optical properties of sol-gel processed PMMA/SiO2 hybrid monoliths. J Non-Cryst Solids 353(29):2807–2812
Rubinstein MP, Sud S (1999) The use of hybrid lenses in management of the irregular cornea. Contact Lens Anterior Eye 22(3):87–90
Gross S, Camozzo D, Di Noto V, Armelao L, Tondello E (2007) PMMA: a key macromolecular component for dielectric low-[kappa] hybrid inorganic-organic polymer films. Eur Polym J 43(3):673–696
Lamaka SV, Montemor MF, Galio AF, Zheludkevich ML, Trindade C, Dick LF, Ferreira MGS (2008) Novel hybrid sol-gel coatings for corrosion protection of AZ31B magnesium alloy. Electrochim Acta 53(14):4773–4783
Banet P, Legagneux L, Hesemann P, Moreau JJE, Nicole L, Quach A, Sanchez C, Tran-Thi TH (2008) Hybrid mesostructured thin films functionalized with DBM as new selective sensors of BF3. Sens Actuators, B 130(1):1–8
Santos JC, Paula AV, Rocha CGF, Nunes GFM, de Castro HF (2008) Morphological and mechanical properties of hybrid matrices of polysiloxane-polyvinyl alcohol prepared by sol-gel technique and their potential for immobilizing enzyme. J Non-Cryst Solids 354(42–44):4823–4826
Ramadan H, Ghanem A, El-Rassy H (2010) Mercury removal from aqueous solutions using silica, polyacrylamide and hybrid silica-polyacrylamide aerogels. Chem Eng J 159(1–3):107–115
Brinker CJ, Scherer GW (1990) Sol-gel science. The physics and chemistry of sol-gel processing. Academic Press, New York, NY
Pierre AC, Pajonk GM (2002) Chemistry of Aerogels and Their Applications. Chem Rev Wash DC US 102(11):4243–4265
Huesing N, Schwertfeger F, Tappert W, Schubert U (1995) Influence of supercritical drying fluid on structure and properties of organically modified silica aerogels. J Non-Cryst Solids 186:37–43
Novak Z, Habulin M, Krmelj V, Knez Z (2003) Silica aerogels as supports for lipase catalyzed esterifications at sub- and supercritical conditions. J Supercrit Fluids 27(2):169–178
Yoldas BE, Annen MJ, Bostaph J (2000) Chemical engineering of aerogel morphology formed under nonsupercritical conditions for thermal insulation. Chem Mater 12(8):2475–2484
Forest L, Gibiat V, Woignier T (1998) Biot’s theory of acoustic propagation in porous media applied to aerogels and alcogels. J Non-Cryst Solids 225(1):287–292
Cantin M, Casse M, Koch L, Jouan R, Mestran P, Roussel D, Bonnin F, Moutel J, Teichner S (1974) Nucl Instrum Methods 118:177
Zhou B, Wang J, Zhao L, Shen J, Deng Z, Li Y (2000) Preparation of C60-doped silica aerogels and the study of photoluminescence properties. J Vacuum Sci Technol B Microelectron Nanometer Struct 18(4):2001–2004
Reed ST, Ashley CS, Brinker CJ, Walko RJ, Ellefson R, Gill J (1990) Porous optical composites. SPIE 1328:220–229
Eid J, Pierre AC, Baret G (2005) Preparation and characterization of transparent Eu doped Y2O3 aerogel monoliths, for application in luminescence. J Non-Cryst Solids 351(3):218–227
Tsou P (1995) Silica aerogel captures cosmic dust intact. J Non-Cryst Solids 186:415–427
Guise MT, Hosticka B, Earp BC, Norris PM (2001) An experimental investigation of aerosol collection utilizing packed beds of silica aerogel microspheres. J Non-Cryst Solids 285(1–3):317–322
Abramian L, El-Rassy H (2009) Adsorption kinetics and thermodynamics of azo-dye Orange II onto highly porous titania aerogel. Chem Eng J 150(2–3):403–410
Akl J, Ghaddar T, Ghanem A, El-Rassy H (2009) Cobalt ferrite aerogels by epoxide sol-gel addition: efficient catalysts for the hydrolysis of 4-nitrophenyl phosphate. J Mol Catal A: Chem 312(1–2):18–22
Pajonk GM (1991) Aerogel catalysts. Appl Catal 72(2):217–266
El Rassy H, Perrard A, Pierre AC (2004) Application of lipase encapsulated in silica aerogels to a transesterification reaction in hydrophobic and hydrophilic solvents: Bi-Bi Ping-Pong kinetics. J Mol Catal B Enzym 30(3–4):137–150
Buisson P, Pierre AC (2006) Immobilization in quartz fiber felt reinforced silica aerogel improves the activity of Candida rugosa lipase in organic solvents. J Mol Catal B Enzym 39(1–4):77–82
Nassreddine S, Karout A, Lorraine Christ M, Pierre AC (2008) Transesterification of a vegetal oil with methanol catalyzed by a silica fibre reinforced aerogel encapsulated lipase. Appl Catal, A 344(1–2):70–77
Karout A, Chopard C, Pierre AC (2007) Immobilization of a lipoxygenase in silica gels for application in aqueous media. J Mol Catal B Enzym 44(3–4):117–127
Al-Oweini R, El-Rassy H (2009) Synthesis and characterization by FTIR spectroscopy of silica aerogels prepared using several Si(OR)4 and R''Si(OR')3 precursors. J Mol Struct 919(1–3):140–145
Nadargi DY, Rao AV (2009) Methyltriethoxysilane: new precursor for synthesizing silica aerogels. J Alloy Comp 467(1–2):397–404
Husing N, Schubert U, Mezei R, Fratzl P, Riegel B, Kiefer W, Kohler D, Mader W (1999) Formation and Structure of Gel Networks from Si(OEt)4/(MeO)3Si(CH2)3NR'2 Mixtures (NR'2 = NH2 or NHCH2CH2NH2). Chem Mater 11(2):451–457
El Rassy H, Buisson P, Bouali B, Perrard A, Pierre AC (2003) Surface characterization of silica aerogels with different proportions of hydrophobic groups, dried by the CO2 supercritical method. Langmuir 19(2):358–363
Rama Rao GV, Krug ME, Balamurugan S, Xu H, Xu Q, Lopez GP (2002) Synthesis and characterization of silica-poly(N-isopropylacrylamide) hybrid membranes: switchable molecular filters. Chem Mater 14(12):5075–5080
Fu Q, Rama Rao GV, Ward TL, Lu Y, Lopez GP (2007) Thermoresponsive transport through ordered mesoporous Silica/PNIPAAm copolymer membranes and microspheres. Langmuir 23(1):170–174
Zareba-Grodz I, Mista W, Strek W, Bukowska E, Hermanowicz K, Maruszewski K (2004) Synthesis and properties of an inorganic-organic hybrid prepared by the sol-gel method. Opt Mater 26(2):207–211
Durme KV, Van Mele B, Loos W, Du Prez FE (2005) Introduction of silica into thermo-responsive poly(N-isopropyl acrylamide) hydrogels: a novel approach to improve response rates. Polymer 46(23):9851–9862
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60(2):309–319
Barrett EP, Joyner LG, Halenda PP (1951) The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J Am Chem Soc 73(1):373–380
Lowell S, Shields JE, Thomas MA, Thommes M (2004) Characterization of porous solids and powders: surface area, pore size, and density. Kluwer Academic, Dordrecht
Song Y, Nan C-W (2007) Preparation of Ca3Co4O9 by polyacrylamide gel processing and its thermoelectric properties. J Sol-Gel Sci Technol 44(2):139–144
Suzuki A, Yamazaki M, Kobiki Y (1996) Direct observation of polymer gel surfaces by atomic force microscopy. J Chem Phys 104(4):1751–1757
Socrates G (2001) Infrared and Raman characteristic group frequencies: Tables and Charts. 3 edn. John Wiley & Sons
Gopal NO, Narasimhulu KV, Rao JL (2004) EPR, optical, infrared and Raman spectral studies of Actinolite mineral. Spectrochim Acta A Mol Biomol Spectrosc 60(11):2441–2448
Duran A, Fernandez Navarro JM, Casariego P, Joglar A (1986) Optical properties of glass coatings containing Fe and Co. J Non-Cryst Solids 82(1–3):391–399
Bertoluzza A, Fagnano C, Antonietta Morelli M, Gottardi V, Guglielmi M (1982) Raman and infrared spectra on silica gel evolving toward glass. J Non-Cryst Solids 48(1):117–128
Gunzler H, Gremlich H-U (2002) IR Spectroscopy: an introduction. Wiley-VCH
Fan Y, Zhang M, Feng Y-Q (2005) Poly(acrylamide-vinylpyridine-N, N'-methylene bisacrylamide) monolithic capillary for in-tube solid-phase microextraction coupled to high performance liquid chromatography. J Chromatogr A 1099(1–2):84–91
Peng Liu LZ, Zhixing Su (2006) Core/shell SiOx@PAM nanospheres from UV-assisted surface-initiated free radical polymerization. J Appl Polym Sci 100(5):3433–3438
Pomonis PJ, Petrakis DE, Ladavos AK, Kolonia KM, Armatas GS, Sklari SD, Dragani PC, Zarlaha A, Stathopoulos VN, Sdoukos AT (2004) A novel method for estimating the C-values of the BET equation in the whole range 0 < P/P0 < 1 using a Scatchard-type treatment of it. Microporous Mesoporous Mater 69(1–2):97–107
Buisson P, Hernandez C, Pierre M, Pierre AC (2001) Encapsulation of lipases in aerogels. J Non-Cryst Solids 285(1–3):295–302
Tanaka T (1992) Phase transitions of gels. In: Polyelectrolyte gels, vol 480. ACS Symposium Series, pp 1-21
Sindorf DW, Maciel GE (1983) Silicon-29 NMR study of dehydrated/rehydrated silica gel using cross polarization and magic-angle spinning. J Am Chem Soc 105(6):1487–1493
Zhu Z, Jian O, Paillet S, Desbrières J, Grassl B (2007) Hydrophobically modified associating polyacrylamide (HAPAM) synthesized by micellar copolymerization at high monomer concentration. Eur Polym J 43(3):824–834
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramadan, H., Coradin, T., Masse, S. et al. Synthesis and Characterization of Mesoporous Hybrid Silica-Polyacrylamide Aerogels and Xerogels. Silicon 3, 63–75 (2011). https://doi.org/10.1007/s12633-010-9064-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12633-010-9064-5