Abstract
Purpose
The scientific rigour of the conduct and reporting of anesthesiology network meta-analyses (NMAs) is unknown. This systematic review and meta-epidemiological study assessed the methodological and reporting quality of NMAs in anesthesiology.
Methods
We searched four databases, including MEDLINE, PubMed, Embase, and the Cochrane Systematic Reviews Database, for anesthesiology NMAs published from inception to October 2020. We assessed the compliance of NMAs against A Measurement Tool to Assess Systematic Reviews (AMSTAR-2), Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement for Network Meta-Analyses (PRISMA-NMA), and PRISMA checklists. We measured the compliance across various items in AMSTAR-2 and PRISMA checklists and provided recommendations to improve quality.
Results
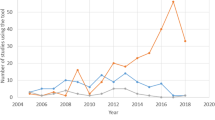
Using the AMSTAR-2 rating method, 84% (52/62) of NMAs were rated “critically low.” Quantitatively, the median [interquartile range] AMSTAR-2 score was 55 [44–69]%, while the PRISMA score was 70 [61–81]%. Methodological and reporting scores showed a strong correlation (R = 0.78). Anesthesiology NMAs had a higher AMSTAR-2 score and PRISMA score if they were published in higher impact factor journals (P = 0.006 and P = 0.01, respectively) or followed PRISMA-NMA reporting guidelines (P = 0.001 and P = 0.002, respectively). Network meta-analyses from China had lower scores (P < 0.001 and P < 0.001, respectively). Neither score improved over time (P = 0.69 and P = 0.67, respectively).
Conclusion
The current study highlights numerous methodological and reporting deficiencies in anesthesiology NMAs. Although the AMSTAR tool has been used to assess the methodological quality of NMAs, dedicated tools for conducting and assessing the methodological quality of NMAs are urgently required.
Study registration
PROSPERO (CRD42021227997); first submitted 23 January 2021.
Résumé
Objectif
La rigueur scientifique de la conduite et de la communication des méta-analyses en réseau (MAR) en anesthésiologie est inconnue. Cette revue systématique et étude méta-épidémiologique a évalué la qualité méthodologique et de communication des MAR en anesthésiologie.
Méthode
Nous avons mené des recherches dans quatre bases de données, soit MEDLINE, PubMed, Embase et la base de données des revues systématiques Cochrane, pour trouver des MAR en anesthésiologie publiées depuis la création de ces bases de données jusqu’en octobre 2020. Nous avons évalué la conformité des MAR par rapport à trois outils, soit : AMSTAR-2 (outil de mesure pour évaluer les revues systématiques), PRISMA-NMA et les listes de contrôle PRISMA. Nous avons mesuré la conformité de divers éléments des listes de contrôle AMSTAR-2 et PRISMA et formulé des recommandations pour améliorer la qualité.
Résultats
En utilisant la méthode de notation AMSTAR-2, 84 % (52/62) des MAR ont reçu la cote « extrêmement faible ». Quantitativement, le score médian [écart interquartile] sur l’AMSTAR-2 était de 55 [44-69] %, tandis que le score PRISMA était de 70 [61-81] %. Les scores méthodologiques et de communication ont montré une forte corrélation (R = 0,78). Les MAR en anesthésiologie avaient un score AMSTAR-2 et un score PRISMA plus élevés si elles étaient publiées dans des revues à facteur d’impact plus élevé (P = 0,006 et P = 0,01, respectivement) ou avaient suivi les lignes directrices de PRISMA-NMA en matière de communication des résultats (P = 0,001 et P = 0,002, respectivement). Les méta-analyses en réseau provenant de Chine avaient des scores plus faibles (P < 0,001 et P < 0,001, respectivement). Aucun des deux scores ne s’est amélioré au fil du temps (P = 0,69 et P = 0,67, respectivement).
Conclusion
La présente étude met en évidence de nombreuses lacunes méthodologiques et de communication dans les MAR en anesthésiologie. Bien que l’outil AMSTAR ait été utilisé pour évaluer la qualité méthodologique des MAR, il est urgent de disposer d’outils spécialisés pour mener des MAR et en évaluer la qualité méthodologique.
Enregistrement de l’étude
PROSPERO (CRD42021227997); soumis pour la première fois le 23 janvier 2021.



Similar content being viewed by others
References
Berlin JA, Golub RM. Meta-analysis as evidence: building a better pyramid. JAMA 2014; 312: 603–5. https://doi.org/10.1001/jama.2014.8167
Lee YH. Strengths and limitations of meta-analysis. Korean J Med 2019; 94: 391–5.
Rouse B, Chaimani A, Li T. Network meta-analysis: an introduction for clinicians. Intern Emerg Med 2017; 12: 103–11. https://doi.org/10.1007/s11739-016-1583-7
Hoaglin DC, Hawkins N, Jansen JP, et al. Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 2. Value Health 2011; 14: 429–37. https://doi.org/10.1016/j.jval.2011.01.011
The Cochrane Collaboration. A network meta-analysis toolkit. Available from URL: https://methods.cochrane.org/cmi/network-meta-analysis-toolkit (accessed January 2023).
Chaimani A, Caldwell DM, Li T, Higgins JP, Salanti G. Undertaking network meta-analyses. In: Higgins PT, Tomas J, Chandler J, et al. (Eds.). Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed. Hoboken: Wiley-Blackwell; 2019: 285–320. https://doi.org/10.1002/9781119536604.ch11
Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015; 4: 1. https://doi.org/10.1186/2046-4053-4-1
Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011; 64: 163–71. https://doi.org/10.1016/j.jclinepi.2010.03.016
Puhan MA, Schünemann HJ, Murad MH, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2014; 349: g5630. https://doi.org/10.1136/bmj.g5630
Hutton B, Catalá-López F, Moher D. The PRISMA statement extension for systematic reviews incorporating network meta-analysis: PRISMA-NMA [Spanish]. Med Clin (Barc) 2016; 147: 262–6. https://doi.org/10.1016/j.medcli.2016.02.025
Liu H, Zhou X, Yu G, Sun X. The effects of the PRISMA statement to improve the conduct and reporting of systematic reviews and meta-analyses of nursing interventions for patients with heart failure. Int J Nurs Pract 2019; 25: e12729. https://doi.org/10.1111/ijn.12729
Nawijn F, Ham WH, Houwert RM, Groenwold RH, Hietbrink F, Smeeing DP. Quality of reporting of systematic reviews and meta-analyses in emergency medicine based on the PRISMA statement. BMC Emerg Med 2019; 19: 19. https://doi.org/10.1186/s12873-019-0233-6
Page MJ, Moher D. Evaluations of the uptake and impact of the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) Statement and extensions: a scoping review. Syst Rev 2017; 6: 263. https://doi.org/10.1186/s13643-017-0663-8
Veroniki AA, Tsokani S, Zevgiti S, et al. Do reporting guidelines have an impact? Empirical assessment of changes in reporting before and after the PRISMA extension statement for network meta-analysis. Syst Rev 2021; 10: 246. https://doi.org/10.1186/s13643-021-01780-9
Salanti G, Higgins JP, Ades AE, Ioannidis JP. Evaluation of networks of randomized trials. Stat Methods Med Res 2008; 17: 279–301. https://doi.org/10.1177/0962280207080643
Ge L, Tian JH, Li XX, et al. Epidemiology characteristics, methodological assessment and reporting of statistical analysis of network meta-analyses in the field of cancer. Sci Rep 2016; 6: 37208. https://doi.org/10.1038/srep37208
Tonin FS, Borba HH, Leonart LP, et al. Methodological quality assessment of network meta-analysis of drug interventions: implications from a systematic review. Int J Epidemiol 2019; 48: 620–32. https://doi.org/10.1093/ije/dyy197
Gao Y, Ge L, Ma X, Shen X, Liu M, Tian J. Improvement needed in the network geometry and inconsistency of Cochrane network meta-analyses: a cross-sectional survey. J Clin Epidemiol 2019; 113: 214–27. https://doi.org/10.1016/j.jclinepi.2019.05.022
Ho RS, Wu X, Yuan J, et al. Methodological quality of meta-analyses on treatments for chronic obstructive pulmonary disease: a cross-sectional study using the AMSTAR (Assessing the Methodological Quality of Systematic Reviews) tool. NPJ Prim Care Respir Med 2015; 25: 14102. https://doi.org/10.1038/npjpcrm.2014.102
Matthias K, Rissling O, Pieper D, et al. The methodological quality of systematic reviews on the treatment of adult major depression needs improvement according to AMSTAR 2: a cross-sectional study. Heliyon 2020; 6: e04776. https://doi.org/10.1016/j.heliyon.2020.e04776
Kanters S, Ford N, Druyts E, Thorlund K, Mills EJ, Bansback N. Use of network meta-analysis in clinical guidelines. Bull World Health Organ 2016; 94: 782–4. https://doi.org/10.2471/blt.16.174326
Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017; 358: j4008. https://doi.org/10.1136/bmj.j4008
Sehmbi H, Retter S, Shah UJ, Nguyen D, Martin J, Uppal V. Epidemiological, methodological, and statistical characteristics of network meta-analysis in anaesthesia: a systematic review. Br J Anaesth 2022; https://doi.org/10.1016/j.bja.2022.08.042
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151: 264–9. https://doi.org/10.7326/0003-4819-151-4-200908180-00135
Schiavo JH. PROSPERO: an international register of systematic review protocols. Med Ref Serv Q 2019; 38: 171–80. https://doi.org/10.1080/02763869.2019.1588072
Covidence. Covidence systematic review software. Available from URL: www.covidence.org (accessed January 2023).
Patil I. Visualizations with statistical details: the 'ggstatsplot' approach. J Open Source Softw 2021; 6: 3167. https://doi.org/10.21105/joss.03167
Schober P, Boer C, Schwarte LA. Correlation coefficients: appropriate use and interpretation. Anesth Analg 2018; 126: 1763–8. https://doi.org/10.1213/ane.0000000000002864
Jüni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA 1999; 282: 1054–60. https://doi.org/10.1001/jama.282.11.1054
Tricco AC, Cogo E, Page MJ, et al. A third of systematic reviews changed or did not specify the primary outcome: a PROSPERO register study. J Clin Epidemiol 2016; 79: 46–54. https://doi.org/10.1016/j.jclinepi.2016.03.025
Dos Santos MBF, Agostini BA, Bassani R, Rocha Pereira GK, Sarkis-Onofre R. Protocol registration improves reporting quality of systematic reviews in dentistry. BMC Med Res Methodol 2020; 20: 57. https://doi.org/10.1186/s12874-020-00939-7
Sterne JA, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366: l4898. https://doi.org/10.1136/bmj.l4898
Lunny C, Tricco AC, Veroniki AA, et al. Methodological review to develop a list of bias items used to assess reviews incorporating network meta-analysis: protocol and rationale. BMJ Open 2021; 11: e045987. https://doi.org/10.1136/bmjopen-2020-045987
Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One 2013; 8: e76654. https://doi.org/10.1371/journal.pone.0076654
Nikolakopoulou A, Higgins JPT, Papakonstantinou T, et al. CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLoS Med 2020; 17: e1003082. https://doi.org/10.1371/journal.pmed.1003082
Chiocchia V, Nikolakopoulou A, Higgins JP, et al. ROB-MEN: a tool to assess risk of bias due to missing evidence in network meta-analysis. BMC Med 2021; 19: 304. https://doi.org/10.1186/s12916-021-02166-3
Sullivan SM, Coyle D, Wells G. What guidance are researchers given on how to present network meta-analyses to end-users such as policymakers and clinicians? A systematic review. PLoS One 2014; 9: e113277. https://doi.org/10.1371/journal.pone.0113277
Higgins J, Lasserson T, Chandler J, et al. Methodological expectations of cochrane intervention reviews (MECIR), 2022. Available from URL: https://community.cochrane.org/mecir-manual (accessed January 2023).
Li T, Puhan MA, Vedula SS, Singh S, Dickersin K. Network meta-analysis-highly attractive but more methodological research is needed. BMC Med 2011; 9: 79. https://doi.org/10.1186/1741-7015-9-79
Lee AW. Use of network meta-analysis in systematic reviews: a survey of authors. Syst Rev 2016; 5: 8. https://doi.org/10.1186/s13643-015-0174-4
Tao KM, Li XQ, Zhou QH, Moher D, Ling CQ, Yu WF. From QUOROM to PRISMA: a survey of high-impact medical journals' instructions to authors and a review of systematic reviews in anesthesia literature. PLoS One 2011; 6: e27611. https://doi.org/10.1371/journal.pone.0027611
Jansen JP. Heterogeneity and subgroup analysis in network meta-analysis. In: Ting N, Cappelleri JC, Ho S, Chen DG (Eds.). Design and Analysis of Subgroups with Biopharmaceutical Applications. Cham: Springer International Publishing; 2020: 369–85.
Dias S, Ades AE, Welton NJ, Jansen JP, Sutton AJ. Meta-regression for relative treatment effects. In: Dias S, Ades AE, Welton NJ, Jansen JP, Sutton J (Ed.). Network Meta‐Analysis for Decision Making. Hoboken: Wiley; 2018: 227–71.
Gwon Y, Mo M, Chen MH, et al. Network meta-regression for ordinal outcomes: applications in comparing Crohn's disease treatments. Stat Med 2020; 39: 1846–70. https://doi.org/10.1002/sim.8518
Rethlefsen ML, Kirtley S, Waffenschmidt S, et al. PRISMA-S: an extension to the PRISMA Statement for reporting literature searches in systematic reviews. Syst Rev 2021; 10: 39. https://doi.org/10.1186/s13643-020-01542-z
Oh JH, Shin WJ, Park S, Chung JS. Reporting and methodologic evaluation of meta-analyses published in the anesthesia literature according to AMSTAR and PRISMA checklists: a preliminary study. Korean J Anesthesiol 2017; 70: 446–55. https://doi.org/10.4097/kjae.2017.70.4.446
Efthimiou O, Mavridis D, Debray TP, et al. Combining randomized and non-randomized evidence in network meta-analysis. Stat Med 2017; 36: 1210–26. https://doi.org/10.1002/sim.7223
Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ 2003; 326: 1167–70. https://doi.org/10.1136/bmj.326.7400.1167
Brignardello-Petersen R, Bonner A, Alexander PE, et al. Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. J Clin Epidemiol 2018; 93: 36–44. https://doi.org/10.1016/j.jclinepi.2017.10.005
Author contributions
Herman Sehmbi, Janet Martin, and Vishal Uppal contributed to study design and protocol. Herman Sehmbi and Ushma J. Shah contributed to initial study screening. Herman Sehmbi, Susanne Retter, Derek Nguyen, and Ushma J. Shah contributed to full-text review and data extraction. Herman Sehmbi and Vishal Uppal contributed to statistical analysis. Herman Sehmbi, Susanne Retter, Ushma J. Shah, Derek Nguyen, Janet Martin, and Vishal Uppal reviewed the manuscript’s final version.
Acknowledgements
The authors would like to thank Darlene Chapman (MLIS), Dalhousie University, for her invaluable help with peer reviewing the literature search for this review.
Disclosures
Dr. Vishal Uppal is an Associate Editor of the Canadian Journal of Anesthesia/Journal canadien d’anesthésie. He had no involvement in the handling of this manuscript.
Funding statement
None.
Editorial responsibility
This submission was handled by Dr. Stephan K. W. Schwarz, Editor-in-Chief, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sehmbi, H., Retter, S., Shah, U.J. et al. Methodological and reporting quality assessment of network meta-analyses in anesthesiology: a systematic review and meta-epidemiological study. Can J Anesth/J Can Anesth 70, 1461–1473 (2023). https://doi.org/10.1007/s12630-023-02510-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-023-02510-6