Abstract
Purpose
Albumin solution is a colloid used for resuscitation in cardiac surgical patients, but it is unclear if it offers advantages over crystalloids. We examined current clinical practice across 11 cardiac surgical centres and the association of albumin with outcomes in a cohort of bleeding cardiac surgical patients.
Methods
This was a post hoc analysis of data from the Effect of Fibrinogen Concentrate vs Cryoprecipitate on Blood Component Transfusion After Cardiac Surgery (FIBRES) trial. Multivariable regression models adjusted for demographic and surgical characteristics were used to examine predictors of early albumin administration (within the initial 24 perioperative hours), late albumin administration (from 24 hr to seven days after cardiopulmonary bypass), and the association of albumin use with 28-day acute kidney injury, mortality, and length of hospital and intensive care unit (ICU) stay.
Results
Of the 735 patients included, 525 (71%) received albumin, ranging from 4.8% to 97.4% of patients across institutions, with 475 (64.6%) receiving albumin early (5% or 25% solution). In the adjusted models, female sex and preoperative hospital admission were associated with early use, while heart failure, female sex, bleeding severity, older age, and prior albumin use were predictors of later administration. Early albumin use was not associated with differences in acute kidney injury (adjusted odds ratio [aOR] 1.77; 95% confidence interval [CI], 0.96 to 3.27; P = 0.07), mortality (aOR 1.66; 95% CI, 0.99 to 2.78; P = 0.05), or length of ICU stay (P = 0.11) or hospital stay (P = 0.67).
Conclusions
Albumin use is common but highly variable within and across sites. Albumin use was not associated with improved outcomes. High quality randomized controlled trials should clarify its role in cardiac surgical patients.
Résumé
Objectif
La solution d’albumine est un colloïde utilisé pour la réanimation des patients chirurgicaux cardiaques, mais nous ne savons pas si elle est avantageuse par rapport aux cristalloïdes. Nous avons examiné la pratique clinique actuelle dans 11 centres de chirurgie cardiaque et l’association entre l’albumine et les devenirs dans une cohorte de patients chirurgicaux cardiaques en état d’hémorragie.
Méthode
Il s’agissait d’une analyse post hoc des données de l’étude FIBRES (Effect of Fibrinogen Concentrate vs Cryoprecipitate on Blood Component Transfusion After Cardiac Surgery, soit ‘Effet du concentré de fibrinogène vs cryoprécipité sur la transfusion de composants sanguins après une chirurgie cardiaque’). Des modèles de régression multivariée ajustés pour tenir compte des caractéristiques démographiques et chirurgicales ont été employés pour examiner les facteurs prédictifs d’une administration précoce d’albumine (dans les premières 24 heures périopératoires), d’une administration tardive d’albumine (entre 24 heures et sept jours après la circulation extracorporelle), et l’association entre l’utilisation d’albumine et l’insuffisance rénale aiguë à 28 jours, la mortalité, et la durée de séjour à l’hôpital et à l’unité de soins intensifs (USI).
Résultats
Parmi les 735 patients inclus, 525 (71 %) ont reçu de l’albumine, allant de 4,8 % à 97,4 % des patients dans tous les établissements, et 475 (64,6 %) ont reçu de l’albumine de manière précoce (solution à 5 % ou 25 %). Dans les modèles ajustés, le sexe féminin et l’admission préopératoire à l’hôpital ont été associés à une utilisation précoce, tandis que l’insuffisance cardiaque, le sexe féminin, la gravité des saignements, un âge plus avancé et l’utilisation antérieure d’albumine étaient des prédicteurs d’une administration tardive. L’utilisation précoce d’albumine n’a pas été associée à des différences en matière d’insuffisance rénale aiguë (rapport de cotes ajusté [RCA] 1,77; intervalle de confiance [IC] à 95 %, 0,96 à 3,27; P = 0,07), de mortalité (RCA = 1,66; IC 95 %, 0,99 à 2,78; P = 0,05), ou de durée de séjour à l’USI (P = 0,11) ou à l’hôpital (P = 0,67).
Conclusion
L’utilisation de l’albumine est fréquente mais très variable au sein des établissements et entre ceux-ci. L’utilisation de l’albumine n’a pas été associée à une amélioration des devenirs. Des études randomisées contrôlées de haute qualité devraient clarifier son rôle chez les patients de chirurgie cardiaque.
Similar content being viewed by others
There has been long-standing debate about fluid management strategies in different patient populations, largely centred on the efficacy of colloids over crystalloids.1,2 Crystalloids are electrolyte solutions, whereas colloids are larger synthetic or natural substances that exert an oncotic pressure facilitating intravascular fluid retention. Colloids may cause less interstitial edema and greater sustained increases in circulating plasma volume than crystalloids do, resulting in lower total fluid administration.3,4 Because of safety concerns regarding the use of synthetic colloids such as hydroxyethyl starches, albumin is the prototypical colloid in Canadian clinical practice.5 Albumin is a natural protein colloid purified from human plasma.3 In Canada, it is distributed through Canadian Blood Services (CBS) and Héma-Québec as a 25% 100 mL (25 g) or 5% 250 or 500 mL (12.5 or 25 g) solution, with the 5% solution exerting a colloid osmotic pressure similar to that of human plasma.3 Despite its wide availability, albumin is expensive (CAD 62 per 25 g dose compared with CAD 2 per 1 L of crystalloid), and has a tenuous supply given that Canada is dependent on imports from other jurisdictions.
In 2009, one fifth of all albumin administered in Canada was to patients undergoing cardiac surgery,6 a patient population where use of albumin is not supported by transfusion medicine experts and CBS because there is no high quality evidence showing benefit over crystalloids.3,6,7 Many of the previously published high quality studies examining the association of albumin with outcomes involve heterogeneous critical care populations, such as patients with sepsis and trauma, thereby limiting generalizability to cardiac surgical patients.8,9,10,11,12,13 In addition, past studies used a range of albumin formulations (4–25%), doses, and resuscitation protocols that do not represent current practices in cardiac surgery.13,14 Additionally, the existing literature examining outcomes associated with albumin use in cardiac surgical patients is conflicting.15,16,17,18,19,20,21
To help elucidate the risk-benefit profile of albumin when used as part of routine practice in cardiac surgery, we undertook a post hoc analysis of data from the Effect of Fibrinogen Concentrate vs Cryoprecipitate on Blood Component Transfusion After Cardiac Surgery (FIBRES) randomized controlled trial22 to examine the association of albumin with clinical outcomes. The population of interest was cardiac surgical patients experiencing clinically significant bleeding, where albumin may be considered a better fluid for resuscitation than crystalloids as it may more quickly restore blood pressure. The aim of this post hoc analysis was to assess current usage patterns and examine the association of albumin with postoperative outcomes in patients undergoing cardiac surgery and experiencing excessive bleeding.
Methods
Data source
This was a post hoc sub-analysis of data from the FIBRES trial, which was conducted at 11 Canadian hospitals from 10 February 2017 to 1 November 2018.22 Institutional Research Ethics Board approval was obtained from the University Health Network and all participating study centres in February 2017. Ethical approval for this substudy, including waiver of written consent for this retrospective analysis, was obtained on 2 June 2020 from the University Health Network Research Ethics Board (Amendment 16-5636.15). This manuscript was prepared according to the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guidelines.23
Population
The FIBRES trial recruited adult patients undergoing cardiac surgery with cardiopulmonary bypass (CPB) for whom fibrinogen replacement was initiated because of clinically significant post-CPB bleeding. Patients were excluded if they had received fibrinogen concentrate or cryoprecipitate within 24 hr of surgery, if they had a severe prior allergic reaction to fibrinogen concentrate or cryoprecipitate, if there was refusal of blood components for religious or other reasons, if there was known pregnancy, or if the plasma fibrinogen level was greater than 3.0 g·L−1 within 30 min of treatment. Of the 827 patients randomized in FIBRES, 735 were treated and included in the primary analysis set (modified intention-to-treat), which consisted of the eligible subpopulation for this study. Fluid management and transfusion practices were not altered from each hospital’s individual established protocols.22 Patients could receive either normal saline or balanced crystalloid (Ringer’s Lactate™ or Plasmalyte™, Baxter Corporation, Mississauga, ON, Canada), albumin, and blood components transfused according to the thresholds of the institution and attending clinicians.22
Predictors and outcomes
Primary analysis
The main predictor of interest was the administration of albumin in the early perioperative period (in the operating room [OR], while on CPB, or in the first 24 hr post-CPB) compared with no administration. Predictors and patient baseline comorbidities were defined as in the FIBRES trial.22 The co-primary outcomes of interest were: 1) all cause 28-day mortality; 2) acute kidney injury (AKI), defined as a greater than two-fold increase in the creatinine level from baseline or a new requirement for dialysis within 28-days of surgery, and 3) return to the OR for re-exploration because of bleeding.
Exploratory analyses
To better assess the underlying differences in patients more likely to be transfused albumin during their perioperative course, we examined potential predictors of early (intraoperatively or within 24 hr of CPB) or late (24 hr to seven days post-CPB) albumin use. Differences in intensive care unit (ICU) and hospital length of stays as well as ICU readmissions were examined between patients receiving and not receiving albumin.
Statistical analysis
Demographic and count data are presented as mean (standard deviations), median [interquartile range], or counts and proportions. The Wilcoxon Rank-Sum test was employed for non-parametric data, t test for parametric data, Fisher’s exact test for categorical data with any contingency table cell counts less than 5, and Chi square statistics for categorical data with cell counts of 5 or greater. Unadjusted effect estimates with 95% confidence intervals (CIs) were obtained from hierarchical generalized estimating equations using a single predictor for the outcome of interest, while adjusted effect estimates with corresponding 95% CIs were obtained from multivariable hierarchical generalized estimating equations. All models accounted for patient clustering by study site. Raw data were examined for missing values, and most variables had < 1% missing data. No variable had more than 5% data missing. Given the low frequency of missing data, complete case analysis was used.
Predictors of early and late albumin use
A series of unadjusted and adjusted hierarchical generalized estimating equation models accounting for patient clustering by site were used to sequentially examine patient characteristics identified in the literature as predictors of early and late albumin administration.20,24 Quasi-likelihood under the Independence Model Criterion (QIC and QICu) values were used to assess models.25
Clinical outcomes
We used unadjusted and adjusted hierarchical generalized estimating equations to model each outcome, accounting for patient clustering by study site. For adjusted clinical outcome models, we specified covariables a priori based on predictors known to be associated with adverse outcomes in cardiac surgical patients in the literature, and tailored to each model to prevent overspecification and multicollinearity.26,27,28 For dichotomous outcomes (mortality, AKI, return to the OR, and ICU readmission), we used multilevel logistic regression models to model the data. We examined count data (length of ICU and hospital stay) for overdispersion and heterogeneity by examining the variance in relation to the mean, as well as Pearson and deviance statistics. Lagrange multiplier statistics were similarly used to test for overdispersion. In the presence of overdispersed count data, we used a negative binomial distribution to model the data. Bleeding severity was adjusted for using the Universal Definition of Perioperative Bleeding (UDPB) score,29 which was scored in FIBRES using a modified definition including postoperative chest drain output, individual units of platelets, plasma, red cells, and factor concentrates transfused, and whether there was a need for surgical re-exploration.22 All models accounted for the original FIBRES trial randomization arm. Quasi-likelihood under the Independence model Criterion (QIC and QICu) values were used to compare models.25 Sensitivity analyses included 1) an examination of any albumin use until seven days post CPB as the predictor of interest, 2) exclusion of patients who received albumin late (between 24 hr and seven days postoperatively) from the primary analysis, and 3) additional adjustment accounting specifically for post-CPB hemodynamic instability. All analyses were conducted with SAS University Edition software (SAS Institute Inc., Cary, NC, USA).
Results
We analyzed data from 735 patients (Fig. 1). Overall, 525 (71%) patients received albumin at least once during the first seven perioperative days. A total of 475 (64.6%) patients received albumin early (intraoperatively or within 24 hr of CPB), 237 (32.2%) received it late (from 24 hr to seven days after cardiopulmonary bypass), and 187 (25.4%) received it during both time periods. There was no difference in original study randomization assignment between groups receiving albumin early (P = 0.83) or at any time (P = 0.96). Compared with the albumin-free group, the cohort receiving albumin at any time was significantly different in baseline demographics and procedural details, including greater burden of pre-existing cardiac comorbidity (Table 1).
Patient flow in this substudy. CPB = cardiopulmonary bypass; FIBRES = the Effect of Fibrinogen Concentrate vs Cryoprecipitate on Blood Component Transfusion After Cardiac Surgery trial.22
Albumin use patterns
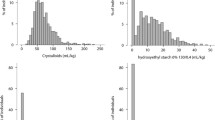
Of the 525 patients who received albumin at any time during the seven-day perioperative period, 127 (24.6%) received it before the end of CPB, 428 (82.8%) received it within 24 hr of CPB, and 235 (45.5%) received it thereafter. Mean and median doses by time period are shown in Table 2, with dose distributions in Fig. 2. The site with the lowest number of albumin transfusions had only two of 42 (4.8%) patients receive albumin, while the site with highest number had 38 of 39 (97.4%) patients who received albumin (Table 3, P < 0.001). In all study sites except for one, the proportion of patients receiving albumin at any time perioperatively was ≥ 50% (Table 3).
Total volume of albumin administration by patient and albumin type in relation to cardiopulmonary bypass. Total patients receiving any albumin product before cardiopulmonary bypass end to seven days post, n = 525 of the initial 735 patients included in the main FIBRES analysis. FIBRES = the Effect of Fibrinogen Concentrate vs Cryoprecipitate on Blood Component Transfusion After Cardiac Surgery trial.22
Predictors of early and late albumin use
Variables associated with early albumin use (intraoperatively until 24 hr after CPB) in the final multivariable model included female sex (adjusted odds ratio [aOR] for male sex with female as reference, 0.70; 95% CI, 0.59 to 0.83; P < 0.01), and preoperative hospitalization (aOR, 1.40; 95% CI, 1.10 to 1.79; P < 0.01) (Electronic Supplementary Material [ESM] eTable 1). Study centre accounted for only 5.4% of the variability in early albumin use, with 94.6% of the variability resulting from within-centre differences in albumin use. Bleeding severity as scored by UDPB category was not predictive of early perioperative albumin administration (ESM eTable 1). For the outcome of late albumin use (from 24 hr to seven days post CPB), variables found to be predictive in the multivariable model included age, sex, early albumin use, UDPB category, decreased preoperative left ventricular ejection fraction, preoperative clinical heart failure, and preoperative hospital admission (ESM eTable 2). For late albumin use, study centre accounted for 1% of the variability in albumin use during this time period, with 99% of the variability related to within-centre differences.
Association of albumin with clinical outcomes
A total of 64 (8.7%) patients experienced mortality within 28 days of surgery, 95 (12.9%) experienced AKI within 28 days of surgery, 121 (16.4%) patients had one surgical re-exploration, and 27 (3.7%) had two or more surgical re-explorations. In unadjusted analyses, there was a significant association between albumin administration and mortality, return to the OR for re-exploration for bleeding, and AKI (Table 4). After adjusting for potential confounders including Class III or IV UDPB severity, albumin did not retain incremental predictive value for mortality or surgical re-exploration (Table 4). Similarly, after adjustment for important confounders such as left ventricular ejection fraction, there was no observed incremental predictive value of albumin administration for AKI (Table 4). These results remained similar in sensitivity analyses; however, the administration of any albumin (intraoperatively to seven days post CPB) was associated with a 183% increase in the odds of 28-day AKI, even when adjusted for important confounders (aOR, 2.83; 95% CI, 1.69 to 4.73; P < 0.01) (ESM eTable 3). In adjusted analyses, early albumin use was not associated with decreases in hospital and ICU length of stay; however, any albumin up to seven days post-CPB was associated with increases in hospital and ICU length of stay (Table 5). There was a modest association of early albumin use with ICU readmission (Table 5).
Discussion
In this study of Canadian cardiac surgical patients experiencing clinically significant bleeding, most patients undergoing care at 11 centres across different provinces were transfused with albumin at least once. At all centres except one, between 50% and 97% of patients received albumin. Nevertheless, in this study, the use of albumin in the early perioperative period did not appear to offer any advantage in terms of improved clinical outcomes. Despite adjusting important confounders, accounting for the initial severity of bleeding, examining differences in the timing of albumin administration, and conducting several sensitivity analyses that accounted for differences in critical illness and postoperative hemodynamic instability, patients who received albumin as an early component of their perioperative resuscitation did not appear to have significantly different outcomes than patients resuscitated using a more restrictive albumin strategy.
The superiority of albumin as a volume expander in cardiac surgical patients has not been definitively established, but its use is primarily guided by its oncotic potential and the belief that it plays a role in preventing interstitial edema and may help maintain endothelial glycocalyx integrity.7,30 In a recent systematic review and meta-analysis of 55 randomized controlled trials (RCTs) involving over 27,000 ICU patients, colloids were found to be more efficacious at maintaining the mean arterial pressure and cardiac index at lower volumes than crystalloids were. Nevertheless, there were no improvements in all-cause or 90-day mortality.10 In many of these large ICU trials, albumin was administered daily with a much larger cumulative dose than what patients undergoing cardiac surgery typically receive.31,9 In our study, 46% of all 5% albumin administered was given as a single 250–500-mL bolus within the initial 24 hr post-CPB. Similarly, 58% of all 25% albumin administered was given as a single bolus of approximately 100 mL. It is unlikely that albumin use at the mostly infrequent, small doses we observed in our study would lead to major clinical benefits if none were seen with regular daily administration and larger cumulative doses in large trials of ICU patients.
Overall, our study suggests there may be few advantages from albumin use, and clinicians do not appear to uniformly agree on albumin’s role as a resuscitation fluid in cardiac surgical patients. This is suggested by the large degree of variability observed in albumin use patterns within and across centres. The lack of a uniform consensus on the role of albumin in this population is likely related to the significant heterogeneity regarding outcomes in the existing literature, where both potential benefits as well as harms related to albumin use have been noted. In our sensitivity analyses examining use of albumin at any time within the first seven perioperative days as a predictor of renal events, albumin was associated with a 183% increase in the odds of postoperative AKI. Although there was no difference in baseline renal function between patients in the albumin and albumin-free groups, the albumin group had a higher proportion of patients with clinical heart failure and decreased left ventricular function, factors which predispose to AKI and mortality.32 Previous propensity-score analyses have reported a two-fold increased risk of AKI and renal replacement therapy with albumin use in cardiac surgical patients.20,21 There are several potential mechanisms by which this may occur: 1) as intracapillary oncotic pressure increases with the use of hyperoncotic colloids, it may overtake the hydrostatic pressure, thereby decreasing the glomerular filtration rate20; 2) albumin may increase central venous pressure, thereby increasing renal venous pressure (“renal afterload”) and limiting glomerular filtration33; 3) in patients with endothelial dysfunction and high capillary permeability, albumin may redistribute to the interstitial space, raising the interstitial oncotic pressure and reducing the effective intravascular volume. This may be particularly true in patients with heart failure and elevated central venous pressure.34 While the exact association of albumin administration with renal outcomes is unclear, our work and previous literature suggest that albumin use is unlikely to significantly improve postoperative renal outcomes compared with patients who do not receive it.
One of the limitations of our study is a lack of data on the indications for albumin use, which may lead to confounding. Such limitations can only be addressed through well designed RCTs to minimize differences between patient groups. While significant effort and care was taken to minimize the potential effect of between-group differences by adjusting for multiple variables in the final models, residual confounding may persist and minimize our ability to isolate the independent effect of albumin on outcomes.35 For this reason, the association observed between later albumin administration and ICU as well as hospital length of stay should be interpreted with caution. Differences in individual clinician practices with regards to perioperative fluid management and transfusion can be controlled for with an RCT. Further, most surgical centres in our study used albumin. While no significant clustering of adverse outcomes was observed within centres with higher or lower albumin use, there were not enough centres with low albumin use to closely examine this. While our methods accounted for clustering, its presence may increase the occurrence of type I errors.36
The population of interest in this study was cardiac surgical patients experiencing clinically significant bleeding requiring treatment, which is a small subset of all patients undergoing cardiac surgery. Patients at increased risk of perioperative bleeding include those with more complex needs as well as those undergoing higher-risk surgical procedures; thus, generalization of our results to the wider cardiac surgical population should be cautious. Well-designed RCTs addressing the design limitations of existing observational studies should be prioritized in cardiac surgical patients. A European group is currently in the process of completing one of the largest RCTs of albumin use in the general cardiac surgical population to date.37 This trial is highly likely to provide important insights on the role and safety of albumin in cardiac surgical patients; however, it will also have important limitations as a single-centre study recruiting primarily low-risk, elective patients. Additionally, the intervention group will receive 4% albumin as the sole resuscitation fluid during the initial 24 hr in the ICU, which may not represent actual clinical practice in Canada where albumin is more commonly used to supplement crystalloids rather than replace them entirely. Further trials will be needed that are specific to the Canadian context and include the highest risk cardiac surgical patients. While there are little data clarifying the advantages and disadvantages of albumin use in the general cardiac surgical population, there are even less data relevant to the highest risk cardiac surgical patients who tend to receive albumin more frequently, including those undergoing correction of congenital cardiac defects, mechanical circulatory support, and heart transplantation and those with severely decreased ventricular function.
Conclusions
We observed high rates of both 5% and 25% albumin administration across Canadian cardiac surgical centres. Nevertheless, we did not observe an improvement in important clinical outcomes between patients who received albumin as part of their perioperative resuscitation and those who did not receive it. Given the lack of high-quality evidence supporting albumin use in this population, the significant variability in its use, and the increased economic costs, a large, definitive RCT is warranted to clarify the role of albumin in the cardiac surgical population.
References
Cook D. Is Albumin Safe? N Engl J Med 2004; 350: 2294-6.
McCluskey SA, Bartoszko J. The chloride horse and normal saline cart: the association of crystalloid choice with acid base status and patient outcomes in kidney transplant recipients. Can J Anesth 2020; 67: 403-7.
Clarke G, Yan M. Clinical Guide to Transfusion: Albumin 2018. Available from URL: https://professionaleducation.blood.ca/en/transfusion/guide-clinique/albumin (accessed June 2021).
Grundmann R, Heistermann S. Postoperative albumin infusion therapy based on colloid osmotic pressure. A prospectively randomized trial. Arch Surg 1985; 120: 911-5.
Barron ME, Wilkes MM, Navickis RJ. A systematic review of the comparative safety of colloids. Arch Surg 2004; 139: 552-63.
King WS, Roland K, Selin S, Chipperfield K, Morrison D. Introduction of guidelines for the use of albumin and the effect on albumin prescribing practices in British Columbia. BC Medical Journal 2012; 54: 34-8.
Aronson S, Nisbet P, Bunke M. Fluid resuscitation practices in cardiac surgery patients in the USA: a survey of health care providers. Perioper Med (Lond) 2017; DOI: https://doi.org/10.1186/s13741-017-0071-6.
Cochrane Injuries Group Albumin Reviewers. Human albumin administration in critically ill patients: systematic review of randomised controlled trials. BMJ 1998; 317: 235-40.
Finfer S, Bellomo R, Boyce N, et al. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med 2004; 350: 2247-56.
Martin GS, Bassett P. Crystalloids vs. colloids for fluid resuscitation in the Intensive care unit: a systematic review and meta-analysis. J Crit Care 2019; 50: 144-54.
Perel P, Roberts I. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev 2011; DOI: https://doi.org/10.1002/14651858.cd000567.pub4d000567.
Roberts I, Blackhall K, Alderson P, Bunn F, Schierhout G. Human albumin solution for resuscitation and volume expansion in critically ill patients. Cochrane Database Syst Rev 2011; DOI: https://doi.org/10.1002/14651858.cd001208.pub4.
Caironi P, Tognoni G, Masson S, et al. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med 2014; 370: 1412-21.
Vincent JL, Russell JA, Jacob M, et al. Albumin administration in the acutely ill: what is new and where next? Crit Care 2014; DOI: https://doi.org/10.1186/cc13991.
Kingeter AJ, Raghunathan K, Munson SH, et al. Association between albumin administration and survival in cardiac surgery: a retrospective cohort study. Can J Anesth 2018; 65: 1218-27.
Lee EH, Kim WJ, Kim JY, et al. Effect of exogenous albumin on the incidence of postoperative acute kidney injury in patients undergoing off-pump coronary artery bypass surgery with a preoperative albumin level of less than 4.0 g/dl. Anesthesiology 2016; 124: 1001-11.
Skhirtladze K, Base EM, Lassnigg A, et al. Comparison of the effects of albumin 5%, hydroxyethyl starch 130/0.4 6%, and Ringer’s lactate on blood loss and coagulation after cardiac surgery. Br J Anaesth 2014; 112: 255-64.
Rasmussen KC, Hojskov M, Johansson PI, et al. Impact of albumin on coagulation competence and hemorrhage during major surgery: a randomized controlled trial. Medicine (Baltimore) 2016; DOI: https://doi.org/10.1097/md.0000000000002720.
Rasmussen KC, Secher NH, Pedersen T. Effect of perioperative crystalloid or colloid fluid therapy on hemorrhage, coagulation competence, and outcome: a systematic review and stratified meta-analysis. Medicine (Baltimore) 2016; DOI: https://doi.org/10.1097/md.0000000000004498.
Frenette AJ, Bouchard J, Bernier P, et al. Albumin administration is associated with acute kidney injury in cardiac surgery: a propensity score analysis. Crit Care 2014; DOI: https://doi.org/10.1186/s13054-014-0602-1.
Ryhammer PK, Tang M, Hoffmann-Petersen J, et al. Colloids in cardiac surgery-friend or foe? J Cardiothorac Vasc Anesth 2017; 31: 1639-48.
Callum J, Farkouh ME, Scales DC, et al. Effect of fibrinogen concentrate vs cryoprecipitate on blood component transfusion after cardiac surgery. The FIBRES randomized clinical trial. JAMA 2019; DOI: https://doi.org/10.1001/jama.2019.17312.
von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370: 1453-7.
Matebele MP, Ramanan M, Thompson K, Cornmell G, Naidoo RV, Shekar K. Albumin use after cardiac surgery. Crit Care Explor 2020; DOI: https://doi.org/10.1097/cce.0000000000000164.
Pan W. Akaike’s information criterion in generalized estimating equations. Biometrics 2001; 57: 120-5.
Bartoszko J, Wijeysundera DN, Karkouti K, et al. Comparison of two major perioperative bleeding scores for cardiac surgery trials: universal definition of Perioperative Bleeding in Cardiac Surgery and European Coronary Artery Bypass Grafting Bleeding Severity Grade. Anesthesiology 2018; 129: 1092-100.
Roques F, Nashef SA, Michel P, et al. Risk factors and outcome in European cardiac surgery: analysis of the EuroSCORE multinational database of 19030 patients. Eur J Cardiothorac Surg 1999; 15: 816-23.
Carr BM, Grover FL, Shroyer AL. Risk factors adversely impacting post coronary artery bypass grafting longer-term vs. shorter-term clinical outcomes. Vessel Plus 2020; DOI: https://doi.org/10.20517/2574-1209.2020.01.
Dyke C, Aronson S, Dietrich W, et al. Universal definition of perioperative bleeding in adult cardiac surgery. J Thorac Cardiovasc Surg 2014; 147: 1458-63.e1.
Moret E, Jacob MW, Ranucci M, Schramko AA. Albumin-beyond fluid replacement in cardiopulmonary bypass surgery: why, how, and when? Semin Cardiothorac Vasc Anesth 2014; 18: 252-9.
Annane D, Siami S, Jaber S, et al. Effects of fluid resuscitation with colloids vs crystalloids on mortality in critically ill patients presenting with hypovolemic shock: the CRISTAL randomized trial. JAMA 2013; 310: 1809-17.
Nadim MK, Forni LG, Bihorac A, et al. Cardiac and vascular surgery-associated acute kidney injury: the 20th International Consensus Conference of the ADQI (Acute Disease Quality Initiative) Group. J Am Heart Assoc 2018; DOI: https://doi.org/10.1161/jaha.118.008834.
Rezende A, Camara L, Leme A, et al. Positive fluid balance as a risk factor for mortality and acute kidney injury in vasoplegic shock after cardiac surgery. Crit Care 2015; DOI: https://doi.org/10.1186/cc14271.
Hesse B, Parving HH, Lund-Jacobsen H, Noer I. Transcapillary escape rate of albumin and right atrial pressure in chronic congestive heart failure before and after treatment. Circ Res 1976; 39: 358-62.
Liang W, Zhao Y, Lee AH. An investigation of the significance of residual confounding effect. Biomed Res Int 2014; DOI: https://doi.org/10.1151/2014/658056.
Barker D, D’Este C, Campbell MJ, McElduff P. Minimum number of clusters and comparison of analysis methods for cross sectional stepped wedge cluster randomised trials with binary outcomes: a simulation study. Trials 2017; DOI: https://doi.org/10.1186/s13063-017-1862-2.
Vlasov H, Juvonen T, Hiippala S, et al. Effect and safety of 4% albumin in the treatment of cardiac surgery patients: study protocol for the randomized, double-blind, clinical ALBICS (ALBumin In Cardiac Surgery) trial. Trials 2020; DOI: https://doi.org/10.1186/s13063-020-4160-3.
Levey AS, Inker LA, Coresh J. GFR estimation: from physiology to public health. Am J Kidney Dis 2014; 63: 820-34.
Author contributions
All authors contributed to manuscript concept, content, writing, and final review.
Disclosures
Justyna Bartoszko, MD MSc: In part supported by a Merit Award from the Department of Anesthesia and Pain Medicine, University of Toronto. Keyvan Karkouti, MD, MSc: In part supported by a Merit Award from the Department of Anesthesia and Pain Medicine, University of Toronto; has received research support, honoraria, or consultancy for speaking engagements from Octapharma, Instrumentation Laboratory, and Bayer. Jeannie Callum, MD: Has received research support from CSL Behring, Octapharma and Canadian Blood Services. Stuart McCluskey: Has received honoraria from Octapharma, Instrumentation Laboratory, and Grifols.
Funding statement
None.
Editorial responsibility
This submission was handled by Dr. Stephan K.W. Schwarz, Editor-in-Chief, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hanley, C., Callum, J., McCluskey, S. et al. Albumin use in bleeding cardiac surgical patients and associated patient outcomes. Can J Anesth/J Can Anesth 68, 1514–1526 (2021). https://doi.org/10.1007/s12630-021-02070-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-021-02070-7